A conserved NR5A1-responsive enhancer regulates SRY in testis-determination
- PMID: 38555298
- PMCID: PMC10981742
- DOI: 10.1038/s41467-024-47162-2
A conserved NR5A1-responsive enhancer regulates SRY in testis-determination
Abstract
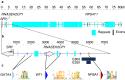
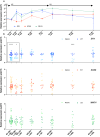
The Y-linked SRY gene initiates mammalian testis-determination. However, how the expression of SRY is regulated remains elusive. Here, we demonstrate that a conserved steroidogenic factor-1 (SF-1)/NR5A1 binding enhancer is required for appropriate SRY expression to initiate testis-determination in humans. Comparative sequence analysis of SRY 5' regions in mammals identified an evolutionary conserved SF-1/NR5A1-binding motif within a 250 bp region of open chromatin located 5 kilobases upstream of the SRY transcription start site. Genomic analysis of 46,XY individuals with disrupted testis-determination, including a large multigenerational family, identified unique single-base substitutions of highly conserved residues within the SF-1/NR5A1-binding element. In silico modelling and in vitro assays demonstrate the enhancer properties of the NR5A1 motif. Deletion of this hemizygous element by genome-editing, in a novel in vitro cellular model recapitulating human Sertoli cell formation, resulted in a significant reduction in expression of SRY. Therefore, human NR5A1 acts as a regulatory switch between testis and ovary development by upregulating SRY expression, a role that may predate the eutherian radiation. We show that disruption of an enhancer can phenocopy variants in the coding regions of SRY that cause human testis dysgenesis. Since disease causing variants in enhancers are currently rare, the regulation of gene expression in testis-determination offers a paradigm to define enhancer activity in a key developmental process.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Identification of a New Enhancer That Promotes Sox9 Expression by a Comparative Analysis of Mouse and Sry-Deficient Amami Spiny Rat.Cytogenet Genome Res. 2023;163(5-6):307-316. doi: 10.1159/000536408. Epub 2024 Jan 20. Cytogenet Genome Res. 2023. PMID: 38246151
-
Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer.Nature. 2008 Jun 12;453(7197):930-4. doi: 10.1038/nature06944. Epub 2008 May 4. Nature. 2008. PMID: 18454134
-
Failure of SOX9 regulation in 46XY disorders of sex development with SRY, SOX9 and SF1 mutations.PLoS One. 2011 Mar 11;6(3):e17751. doi: 10.1371/journal.pone.0017751. PLoS One. 2011. PMID: 21412441 Free PMC article.
-
SRY: A transcriptional activator of mammalian testis determination.Int J Biochem Cell Biol. 2010 Mar;42(3):417-20. doi: 10.1016/j.biocel.2009.12.005. Epub 2009 Dec 18. Int J Biochem Cell Biol. 2010. PMID: 20005972 Review.
-
Sry and Sox9: mammalian testis-determining genes.Cell Mol Life Sci. 1999 Jun;55(6-7):839-56. doi: 10.1007/pl00013200. Cell Mol Life Sci. 1999. PMID: 10412367 Free PMC article. Review.
References
-
- Stevant, I. & Nef, S. Genetic control of gonadal sex determination and development. Trends Genet.10.1016/j.tig.2019.02.004 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


