Designer cell therapy for tissue regeneration
- PMID: 38491394
- PMCID: PMC10941617
- DOI: 10.1186/s41232-024-00327-4
Designer cell therapy for tissue regeneration
Abstract
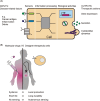
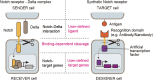
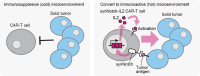
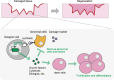
Cancer cell therapy, particularly chimeric antigen receptor (CAR) T-cell therapy for blood cancers, has emerged as a powerful new modality for cancer treatment. Therapeutic cells differ significantly from conventional drugs, such as small molecules and biologics, as they possess cellular information processing abilities to recognize and respond to abnormalities in the body. This capability enables the targeted delivery of therapeutic factors to specific locations and times. Various types of designer cells have been developed and tested to overcome the shortcomings of CAR T cells and expand their functions in the treatment of solid tumors. In particular, synthetic receptor technologies are a key to designing therapeutic cells that specifically improve tumor microenvironment. Such technologies demonstrate great potential for medical applications to regenerate damaged tissues as well that are difficult to cure with conventional drugs. In this review, we introduce recent developments in next-generation therapeutic cells for cancer treatment and discuss the application of designer therapeutic cells for tissue regeneration.
Keywords: Cell engineering; Cell therapy; Synthetic receptor; Tissue microenvironment; Tissue regeneration.
© 2024. The Author(s).
Conflict of interest statement
S.T. is an inventor on a patent for synthetic Notch receptors (Patent No.: US 10,590,182 B2) held by the Regents of the University of California, which is licensed to Gilead. N.Z. declares no competing interests.
Figures
Similar articles
-
Secretory co-factors in next-generation cellular therapies for cancer.Front Immunol. 2022 Aug 18;13:907022. doi: 10.3389/fimmu.2022.907022. eCollection 2022. Front Immunol. 2022. PMID: 36059449 Free PMC article. Review.
-
Challenges and Prospects of Chimeric Antigen Receptor T-cell Therapy for Metastatic Prostate Cancer.Eur Urol. 2020 Mar;77(3):299-308. doi: 10.1016/j.eururo.2019.08.014. Epub 2019 Aug 28. Eur Urol. 2020. PMID: 31471138 Review.
-
Better by design: What to expect from novel CAR-engineered cell therapies?Biotechnol Adv. 2022 Sep;58:107917. doi: 10.1016/j.biotechadv.2022.107917. Epub 2022 Feb 9. Biotechnol Adv. 2022. PMID: 35149146 Review.
-
Chimeric antigen receptor-engineered T-cell therapy for liver cancer.Hepatobiliary Pancreat Dis Int. 2018 Aug;17(4):301-309. doi: 10.1016/j.hbpd.2018.05.005. Epub 2018 May 24. Hepatobiliary Pancreat Dis Int. 2018. PMID: 29861325 Review.
-
Chimeric antigen receptor T cells applied to solid tumors.Front Immunol. 2022 Oct 31;13:984864. doi: 10.3389/fimmu.2022.984864. eCollection 2022. Front Immunol. 2022. PMID: 36389701 Free PMC article. Review.
References
-
- Hamieh M, Mansilla-Soto J, Riviere I, Sadelain M. Programming CAR T cell tumor recognition: tuned antigen sensing and logic gating. Cancer Discov. 2023;13(4):829–843. doi: 10.1158/2159-8290.CD-23-0101. - DOI - PMC - PubMed
Publication types
Grants and funding
- JPMJPR2147/Precursory Research for Embryonic Science and Technology
- 21H05291/Japan Society for the Promotion of Science
- 23ama221318s0101/Japan Agency for Medical Research and Development
- 22bm0704048h0003/Japan Agency for Medical Research and Development
- World Premier International Research Center Initiative (WPI)/Ministry of Education, Culture, Sports, Science and Technology
LinkOut - more resources
Full Text Sources


