Development and Assessment of SNP Genotyping Arrays for Citrus and Its Close Relatives
- PMID: 38475537
- PMCID: PMC10935022
- DOI: 10.3390/plants13050691
Development and Assessment of SNP Genotyping Arrays for Citrus and Its Close Relatives
Abstract
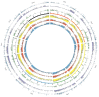
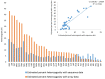
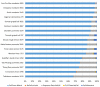
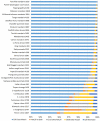
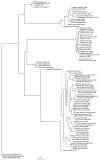
Rapid advancements in technologies provide various tools to analyze fruit crop genomes to better understand genetic diversity and relationships and aid in breeding. Genome-wide single nucleotide polymorphism (SNP) genotyping arrays offer highly multiplexed assays at a relatively low cost per data point. We report the development and validation of 1.4M SNP Axiom® Citrus HD Genotyping Array (Citrus 15AX 1 and Citrus 15AX 2) and 58K SNP Axiom® Citrus Genotyping Arrays for Citrus and close relatives. SNPs represented were chosen from a citrus variant discovery panel consisting of 41 diverse whole-genome re-sequenced accessions of Citrus and close relatives, including eight progenitor citrus species. SNPs chosen mainly target putative genic regions of the genome and are accurately called in both Citrus and its closely related genera while providing good coverage of the nuclear and chloroplast genomes. Reproducibility of the arrays was nearly 100%, with a large majority of the SNPs classified as the most stringent class of markers, "PolyHighResolution" (PHR) polymorphisms. Concordance between SNP calls in sequence data and array data average 98%. Phylogenies generated with array data were similar to those with comparable sequence data and little affected by 3 to 5% genotyping error. Both arrays are publicly available.
Keywords: Citrus; genotyping array; germplasm; plant breeding; single nucleotide polymorphism.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Development and validation of the Axiom(®) Apple480K SNP genotyping array.Plant J. 2016 Apr;86(1):62-74. doi: 10.1111/tpj.13145. Plant J. 2016. PMID: 26919684
-
Development of a highly efficient Axiom™ 70 K SNP array for Pyrus and evaluation for high-density mapping and germplasm characterization.BMC Genomics. 2019 May 2;20(1):331. doi: 10.1186/s12864-019-5712-3. BMC Genomics. 2019. PMID: 31046664 Free PMC article.
-
Development of an integrated 200K SNP genotyping array and application for genetic mapping, genome assembly improvement and genome wide association studies in pear (Pyrus).Plant Biotechnol J. 2019 Aug;17(8):1582-1594. doi: 10.1111/pbi.13085. Epub 2019 Feb 17. Plant Biotechnol J. 2019. PMID: 30690857 Free PMC article.
-
The Wheat 660K SNP array demonstrates great potential for marker-assisted selection in polyploid wheat.Plant Biotechnol J. 2020 Jun;18(6):1354-1360. doi: 10.1111/pbi.13361. Epub 2020 Mar 10. Plant Biotechnol J. 2020. PMID: 32065714 Free PMC article. Review.
-
Large SNP arrays for genotyping in crop plants.J Biosci. 2012 Nov;37(5):821-8. doi: 10.1007/s12038-012-9225-3. J Biosci. 2012. PMID: 23107918 Review.
References
-
- FAO . FAO Statistical Yearbook: World Food and Agriculture. FAO; Roma, Italy: 2013.
-
- FAO FAOstat. 2020. [(accessed on 23 December 2023)]. Available online: https://www.fao.org/faostat/
-
- United Nations . Food and Agriculture Organization of the United Nations (FAO) In: United Nations, editor. Yearbook of the United Nations 1995 (UN) United Nations; New York, NY, USA: 1995. pp. 1469–1472.
-
- Tripoli E., Guardia M.L., Giammanco S., Majo D.D., Giammanco M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007;104:466–479. doi: 10.1016/j.foodchem.2006.11.054. - DOI
Grants and funding
- 2013-67013-21110 /project accession no. 1001031/NIFA Plant Biology/Plant Breeding Program
- 2018-70016-27453/ project accession no. 1014825/NIFA Citrus Disease Research and Extension Program
- NRSP-10/USDA National Institute of Food and Agriculture
- DE-AC02-05CH11231/Office of Science of the US Department of Energy
LinkOut - more resources
Full Text Sources


