Increased reliance on temporal coding when target sound is softer than the background
- PMID: 38396044
- PMCID: PMC10891139
- DOI: 10.1038/s41598-024-54865-5
Increased reliance on temporal coding when target sound is softer than the background
Abstract
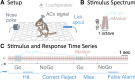
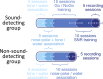
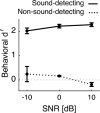
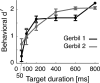
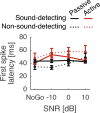
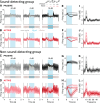
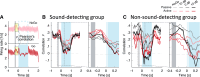
Everyday environments often contain multiple concurrent sound sources that fluctuate over time. Normally hearing listeners can benefit from high signal-to-noise ratios (SNRs) in energetic dips of temporally fluctuating background sound, a phenomenon called dip-listening. Specialized mechanisms of dip-listening exist across the entire auditory pathway. Both the instantaneous fluctuating and the long-term overall SNR shape dip-listening. An unresolved issue regarding cortical mechanisms of dip-listening is how target perception remains invariant to overall SNR, specifically, across different tone levels with an ongoing fluctuating masker. Equivalent target detection over both positive and negative overall SNRs (SNR invariance) is reliably achieved in highly-trained listeners. Dip-listening is correlated with the ability to resolve temporal fine structure, which involves temporally-varying spike patterns. Thus the current work tests the hypothesis that at negative SNRs, neuronal readout mechanisms need to increasingly rely on decoding strategies based on temporal spike patterns, as opposed to spike count. Recordings from chronically implanted electrode arrays in core auditory cortex of trained and awake Mongolian gerbils that are engaged in a tone detection task in 10 Hz amplitude-modulated background sound reveal that rate-based decoding is not SNR-invariant, whereas temporal coding is informative at both negative and positive SNRs.
Keywords: Auditory cortex; Central masking; Dip-listening; Gerbil; Modulation masking release; SNR invariance; Signal-to-noise ratio.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
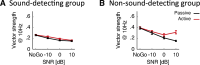
Similar articles
-
The fluctuating masker benefit for normal-hearing and hearing-impaired listeners with equal audibility at a fixed signal-to-noise ratio.J Acoust Soc Am. 2019 Apr;145(4):2113. doi: 10.1121/1.5096641. J Acoust Soc Am. 2019. PMID: 31046298 Free PMC article.
-
Impact of stimulus-related factors and hearing impairment on listening effort as indicated by pupil dilation.Hear Res. 2017 Aug;351:68-79. doi: 10.1016/j.heares.2017.05.012. Epub 2017 May 25. Hear Res. 2017. PMID: 28622894
-
Auditory and auditory-visual intelligibility of speech in fluctuating maskers for normal-hearing and hearing-impaired listeners.J Acoust Soc Am. 2009 May;125(5):3358-72. doi: 10.1121/1.3110132. J Acoust Soc Am. 2009. PMID: 19425676
-
Assessing and Modeling Spatial Release From Listening Effort in Listeners With Normal Hearing: Reference Ranges and Effects of Noise Direction and Age.Trends Hear. 2022 Jan-Dec;26:23312165221129407. doi: 10.1177/23312165221129407. Trends Hear. 2022. PMID: 36285532 Free PMC article. Review.
-
Enhancing Auditory Selective Attention Using a Visually Guided Hearing Aid.J Speech Lang Hear Res. 2017 Oct 17;60(10):3027-3038. doi: 10.1044/2017_JSLHR-H-17-0071. J Speech Lang Hear Res. 2017. PMID: 29049603 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources


