Advancements and future prospects of adeno-associated virus-mediated gene therapy for sensorineural hearing loss
- PMID: 38327848
- PMCID: PMC10847333
- DOI: 10.3389/fnins.2024.1272786
Advancements and future prospects of adeno-associated virus-mediated gene therapy for sensorineural hearing loss
Abstract
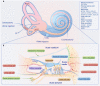
Sensorineural hearing loss (SNHL), a highly prevalent sensory impairment, results from a multifaceted interaction of genetic and environmental factors. As we continually gain insights into the molecular basis of auditory development and the growing compendium of deafness genes identified, research on gene therapy for SNHL has significantly deepened. Adeno-associated virus (AAV), considered a relatively secure vector for gene therapy in clinical trials, can deliver various transgenes based on gene therapy strategies such as gene replacement, gene silencing, gene editing, or gene addition to alleviate diverse types of SNHL. This review delved into the preclinical advances in AAV-based gene therapy for SNHL, spanning hereditary and acquired types. Particular focus is placed on the dual-AAV construction method and its application, the vector delivery route of mouse inner ear models (local, systemic, fetal, and cerebrospinal fluid administration), and the significant considerations in transforming from AAV-based animal model inner ear gene therapy to clinical implementation.
Keywords: adeno-associated virus; delivery route; dual-AAV; gene therapy; sensorineural hearing loss.
Copyright © 2024 Li, Shen, Liu, Qi and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dual and triple AAV delivery of large therapeutic gene sequences into the inner ear.Hear Res. 2020 Sep 1;394:107912. doi: 10.1016/j.heares.2020.107912. Epub 2020 Feb 10. Hear Res. 2020. PMID: 32067799 Review.
-
Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV.Mol Ther. 2017 Feb 1;25(2):379-391. doi: 10.1016/j.ymthe.2016.12.010. Epub 2017 Jan 9. Mol Ther. 2017. PMID: 28082074 Free PMC article.
-
Adeno-associated virus gene replacement for recessive inner ear dysfunction: Progress and challenges.Hear Res. 2020 Sep 1;394:107947. doi: 10.1016/j.heares.2020.107947. Epub 2020 Mar 18. Hear Res. 2020. PMID: 32247629 Free PMC article. Review.
-
Gene Therapy for Human Sensorineural Hearing Loss.Front Cell Neurosci. 2019 Jul 16;13:323. doi: 10.3389/fncel.2019.00323. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31379508 Free PMC article. Review.
-
Autosomal Recessive Non-Syndromic Deafness: Is AAV Gene Therapy a Real Chance?Audiol Res. 2024 Feb 22;14(2):239-253. doi: 10.3390/audiolres14020022. Audiol Res. 2024. PMID: 38525683 Free PMC article. Review.
Cited by
-
Dynamic micro-optical coherence tomography enables structural and metabolic imaging of the mammalian cochlea.Front Mol Neurosci. 2024 Oct 10;17:1436837. doi: 10.3389/fnmol.2024.1436837. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39449964 Free PMC article.
-
Advancements and challenges in stem cell transplantation for regenerative medicine.Heliyon. 2024 Aug 10;10(16):e35836. doi: 10.1016/j.heliyon.2024.e35836. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39247380 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials


