Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders
- PMID: 38256124
- PMCID: PMC10816966
- DOI: 10.3390/ijms25021050
Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders
Abstract
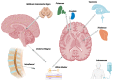
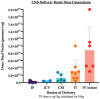
Genetic disorders of the central nervous system (CNS) comprise a significant portion of disability in both children and adults. Several preclinical animal models have shown effective adeno-associated virus (AAV) mediated gene transfer for either treatment or prevention of autosomal recessive genetic disorders. Owing to the intricacy of the human CNS and the blood-brain barrier, it is difficult to deliver genes, particularly since the expression of any given gene may be required in a particular CNS structure or cell type at a specific time during development. In this review, we analyzed delivery methods for AAV-mediated gene therapy in past and current clinical trials. The delivery routes analyzed were direct intraparenchymal (IP), intracerebroventricular (ICV), intra-cisterna magna (CM), lumbar intrathecal (IT), and intravenous (IV). The results demonstrated that the dose used in these routes varies dramatically. The average total doses used were calculated and were 1.03 × 1013 for IP, 5.00 × 1013 for ICV, 1.26 × 1014 for CM, and 3.14 × 1014 for IT delivery. The dose for IV delivery varies by patient weight and is 1.13 × 1015 IV for a 10 kg infant. Ultimately, the choice of intervention must weigh the risk of an invasive surgical procedure to the toxicity and immune response associated with a high dose vector.
Keywords: AAV; adeno-associated virus vector; gene delivery; gene therapy; inherited disorders.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Biodistribution of Adeno-Associated Virus Gene Therapy Following Cerebrospinal Fluid-Directed Administration.Hum Gene Ther. 2023 Feb;34(3-4):94-111. doi: 10.1089/hum.2022.163. Hum Gene Ther. 2023. PMID: 36606687 Review.
-
A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna.Mol Ther. 2020 Feb 5;28(2):411-421. doi: 10.1016/j.ymthe.2019.11.012. Epub 2019 Nov 16. Mol Ther. 2020. PMID: 31813800 Free PMC article.
-
AAV-PHP.B Administration Results in a Differential Pattern of CNS Biodistribution in Non-human Primates Compared with Mice.Mol Ther. 2019 Nov 6;27(11):2018-2037. doi: 10.1016/j.ymthe.2019.07.017. Epub 2019 Aug 5. Mol Ther. 2019. PMID: 31420242 Free PMC article.
-
Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals.Hum Gene Ther. 2018 Jan;29(1):15-24. doi: 10.1089/hum.2017.026. Epub 2017 Oct 3. Hum Gene Ther. 2018. PMID: 28806897 Free PMC article.
-
Immune responses to central nervous system directed adeno-associated virus gene therapy: Does direct CNS delivery make a difference?Neurotherapeutics. 2024 Jul;21(4):e00435. doi: 10.1016/j.neurot.2024.e00435. Epub 2024 Aug 23. Neurotherapeutics. 2024. PMID: 39180957 Free PMC article. Review.
Cited by
-
Current Treatment Methods for Charcot-Marie-Tooth Diseases.Biomolecules. 2024 Sep 9;14(9):1138. doi: 10.3390/biom14091138. Biomolecules. 2024. PMID: 39334903 Free PMC article. Review.
-
A Novel CRISPR-Cas9 Strategy to Target DYSTROPHIN Mutations Downstream of Exon 44 in Patient-Specific DMD iPSCs.Cells. 2024 Jun 4;13(11):972. doi: 10.3390/cells13110972. Cells. 2024. PMID: 38891104 Free PMC article.
-
Intrathecal gene therapy for neurologic disease in humans.Mol Ther. 2024 May 1;32(5):1185-1186. doi: 10.1016/j.ymthe.2024.04.013. Epub 2024 Apr 24. Mol Ther. 2024. PMID: 38663405 No abstract available.
References
-
- Tatschin J.-D., West M.H.P., Sandbank T., Carter B.J. A Human Parvovirus, Adeno-Associated Virus, as a Eucaryotic Vector: Transient Expression and Encapsidation of the Procaryotic Gene for Chloramphenicol Acetyltransferase. Mol. Cell. Biol. 1984;4:2072–2081. doi: 10.1128/mcb.4.10.2072-2081.1984. - DOI - PMC - PubMed
-
- Flotte T.R., Afione S.A., Conrad C., McGrath S.A., Solow R., Oka H., Zeitlin P.L., Guggino W.B., Carter B.J. Stable in Vivo Expression of the Cystic Fibrosis Transmembrane Conductance Regulator with an Adeno-Associated Virus Vector. Proc. Natl. Acad. Sci. USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


