The endometrial microbiota and early pregnancy loss
- PMID: 38195891
- PMCID: PMC10988105
- DOI: 10.1093/humrep/dead274
The endometrial microbiota and early pregnancy loss
Abstract
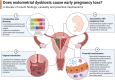
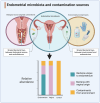
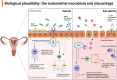
The human endometrium is a dynamic entity that plays a pivotal role in mediating the complex interplay between the mother and developing embryo. Endometrial disruption can lead to pregnancy loss, impacting both maternal physical and psychological health. Recent research suggests that the endometrial microbiota may play a role in this, although the exact mechanisms are still being explored, aided by recent technological advancements and our growing understanding of host immune responses. Suboptimal or dysbiotic vaginal microbiota, characterized by increased microbial diversity and reduced Lactobacillus dominance, has been associated with various adverse reproductive events, including miscarriage. However, the mechanisms linking the lower reproductive tract microbiota with pregnancy loss remain unclear. Recent observational studies implicate a potential microbial continuum between the vaginal and endometrial niche in patients with pregnancy loss; however, transcervical sampling of the low biomass endometrium is highly prone to cross-contamination, which is often not controlled for. In this review, we explore emerging evidence supporting the theory that a dysbiotic endometrial microbiota may modulate key inflammatory pathways required for successful embryo implantation and pregnancy development. We also highlight that a greater understanding of the endometrial microbiota, its relationship with the local endometrial microenvironment, and potential interventions remain a focus for future research.
Keywords: endometrial microbiota; immune microenvironment; inflammation; microbiome; miscarriage; recurrent pregnancy loss.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
P.R.B. and D.A.M. have a patent for the use of
Figures
Similar articles
-
Biologia Futura: endometrial microbiome affects endometrial receptivity from the perspective of the endometrial immune microenvironment.Biol Futur. 2022 Sep;73(3):291-300. doi: 10.1007/s42977-022-00134-3. Epub 2022 Sep 26. Biol Futur. 2022. PMID: 36161422 Review.
-
Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss.Int J Mol Sci. 2024 Jan 3;25(1):622. doi: 10.3390/ijms25010622. Int J Mol Sci. 2024. PMID: 38203793 Free PMC article. Review.
-
Evidence that the endometrial microbiota has an effect on implantation success or failure.Am J Obstet Gynecol. 2016 Dec;215(6):684-703. doi: 10.1016/j.ajog.2016.09.075. Epub 2016 Oct 4. Am J Obstet Gynecol. 2016. PMID: 27717732
-
How uterine microbiota might be responsible for a receptive, fertile endometrium.Hum Reprod Update. 2018 Jul 1;24(4):393-415. doi: 10.1093/humupd/dmy012. Hum Reprod Update. 2018. PMID: 29668899 Review.
-
The first glimpse of the endometrial microbiota in early pregnancy.Am J Obstet Gynecol. 2020 Apr;222(4):296-305. doi: 10.1016/j.ajog.2020.01.031. Epub 2020 Feb 10. Am J Obstet Gynecol. 2020. PMID: 32057732 Free PMC article.
Cited by
-
Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection.Microorganisms. 2024 Aug 10;12(8):1641. doi: 10.3390/microorganisms12081641. Microorganisms. 2024. PMID: 39203483 Free PMC article. Review.
References
-
- Bayar E, MacIntyre DA, Sykes L, Mountain K, Parks T, Lee P, Bennett P.. Safety, tolerability, and acceptability of Lactobacillus crispatus CTV-05 (LACTIN-V) in pregnant women at high-risk of preterm birth. Benef Microbes 2023;14:45–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


