Multi-ancestry genome-wide association meta-analysis of Parkinson's disease
- PMID: 38155330
- PMCID: PMC10786718
- DOI: 10.1038/s41588-023-01584-8
Multi-ancestry genome-wide association meta-analysis of Parkinson's disease
Abstract
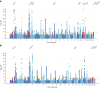
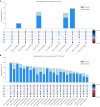
Although over 90 independent risk variants have been identified for Parkinson's disease using genome-wide association studies, most studies have been performed in just one population at a time. Here we performed a large-scale multi-ancestry meta-analysis of Parkinson's disease with 49,049 cases, 18,785 proxy cases and 2,458,063 controls including individuals of European, East Asian, Latin American and African ancestry. In a meta-analysis, we identified 78 independent genome-wide significant loci, including 12 potentially novel loci (MTF2, PIK3CA, ADD1, SYBU, IRS2, USP8, PIGL, FASN, MYLK2, USP25, EP300 and PPP6R2) and fine-mapped 6 putative causal variants at 6 known PD loci. By combining our results with publicly available eQTL data, we identified 25 putative risk genes in these novel loci whose expression is associated with PD risk. This work lays the groundwork for future efforts aimed at identifying PD loci in non-European populations.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
K.H. and members of the 23andMe Research Team are employed by and hold stock or stock options in 23andMe. M.A.N.’s participation in this project was part of a competitive contract awarded to Data Tecnica International by the NIH to support open science research; he also currently serves on the scientific advisory board for Clover Therapeutics and is an advisor to Neuron23. A.J.N. reports consultancy and personal fees from AstraZeneca, AbbVie, Profile, Roche, Biogen, UCB, Bial, Charco Neurotech, uMedeor, Alchemab and Britannia outside the submitted work. The other authors declare no competing interests.
Figures

Similar articles
-
Novel Variants Linked to the Prodromal Stage of Parkinson's Disease (PD) Patients.Diagnostics (Basel). 2024 Apr 29;14(9):929. doi: 10.3390/diagnostics14090929. Diagnostics (Basel). 2024. PMID: 38732343 Free PMC article.
-
Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta-analysis of genome-wide association studies.Lancet Neurol. 2019 Dec;18(12):1091-1102. doi: 10.1016/S1474-4422(19)30320-5. Lancet Neurol. 2019. PMID: 31701892 Free PMC article.
-
Genome-wide Association Identifies Novel Etiological Insights Associated with Parkinson's Disease in African and African Admixed Populations.medRxiv [Preprint]. 2023 May 7:2023.05.05.23289529. doi: 10.1101/2023.05.05.23289529. medRxiv. 2023. Update in: Lancet Neurol. 2023 Nov;22(11):1015-1025. doi: 10.1016/S1474-4422(23)00283-1 PMID: 37398408 Free PMC article. Updated. Preprint.
-
Association between ubiquitin carboxy-terminal hydrolase-L1 S18Y variant and risk of Parkinson's disease: the impact of ethnicity and onset age.Neurol Sci. 2015 Feb;36(2):179-88. doi: 10.1007/s10072-014-1987-y. Epub 2014 Nov 6. Neurol Sci. 2015. PMID: 25370916 Review.
-
Lack of association between UCHL1 S18Y gene polymorphism and Parkinson's disease in the Asian population: a meta-analysis.Neurol Sci. 2014 Dec;35(12):1867-76. doi: 10.1007/s10072-014-1973-4. Epub 2014 Oct 30. Neurol Sci. 2014. PMID: 25354657 Review.
Cited by
-
Exome sequencing in Asian populations identifies low-frequency and rare coding variation influencing Parkinson's disease risk.Nat Aging. 2024 Nov 21. doi: 10.1038/s43587-024-00760-7. Online ahead of print. Nat Aging. 2024. PMID: 39572736
-
Plasma acellular transcriptome contains Parkinson's disease signatures that can inform clinical diagnosis.medRxiv [Preprint]. 2024 Oct 18:2024.10.18.24315717. doi: 10.1101/2024.10.18.24315717. medRxiv. 2024. PMID: 39484251 Free PMC article. Preprint.
-
A network-based systems genetics framework identifies pathobiology and drug repurposing in Parkinson's disease.Res Sq [Preprint]. 2024 Oct 14:rs.3.rs-4869009. doi: 10.21203/rs.3.rs-4869009/v1. Res Sq. 2024. PMID: 39483867 Free PMC article. Preprint.
-
The Black and African American Connections to Parkinson's Disease (BLAAC PD) study protocol.BMC Neurol. 2024 Oct 21;24(1):403. doi: 10.1186/s12883-024-03914-7. BMC Neurol. 2024. PMID: 39434044 Free PMC article.
-
Combining Biomarkers with Genetics In Prodromal/Earliest Phase Parkinson's Disease.J Parkinsons Dis. 2024;14(s2):S345-S351. doi: 10.3233/JPD-240155. J Parkinsons Dis. 2024. PMID: 39331107 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


