Sialic Acid Mimetic Microglial Sialic Acid-Binding Immunoglobulin-like Lectin Agonism: Potential to Restore Retinal Homeostasis and Regain Visual Function in Age-Related Macular Degeneration
- PMID: 38139861
- PMCID: PMC10747662
- DOI: 10.3390/ph16121735
Sialic Acid Mimetic Microglial Sialic Acid-Binding Immunoglobulin-like Lectin Agonism: Potential to Restore Retinal Homeostasis and Regain Visual Function in Age-Related Macular Degeneration
Abstract
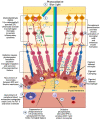
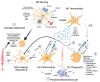
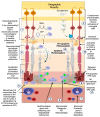
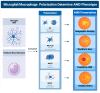
Age-related macular degeneration (AMD), a leading cause of visual loss and dysfunction worldwide, is a disease initiated by genetic polymorphisms that impair the negative regulation of complement. Proteomic investigation points to altered glycosylation and loss of Siglec-mediated glyco-immune checkpoint parainflammatory and inflammatory homeostasis as the main determinant for the vision impairing complications of macular degeneration. The effect of altered glycosylation on microglial maintained retinal para-inflammatory homeostasis and eventual recruitment and polarization of peripheral blood monocyte-derived macrophages (PBMDMs) into the retina can explain the phenotypic variability seen in this clinically heterogenous disease. Restoring glyco-immune checkpoint control with a sialic acid mimetic agonist targeting microglial/macrophage Siglecs to regain retinal para-inflammatory and inflammatory homeostasis is a promising therapeutic that could halt the progression of and improve visual function in all stages of macular degeneration.
Keywords: Siglecs; geographic atrophy; glycosylation; macrophages; macular degeneration; microglia; nanoparticles; polysialic acid; sialic acid.
Conflict of interest statement
The authors (A.K., M.A.G.) are full-time or (M.J.T.) part-time employees of Aviceda Therapeutics, the funder of this paper. A.J.T. and E.M.T. have no conflicts of interest.
Figures

Similar articles
-
PolySialic acid-nanoparticles inhibit macrophage mediated inflammation through Siglec agonism: a potential treatment for age related macular degeneration.Front Immunol. 2023 Nov 16;14:1237016. doi: 10.3389/fimmu.2023.1237016. eCollection 2023. Front Immunol. 2023. PMID: 38045700 Free PMC article.
-
Modulation of immune cell reactivity with cis-binding Siglec agonists.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2012408118. doi: 10.1073/pnas.2012408118. Proc Natl Acad Sci U S A. 2021. PMID: 33431669 Free PMC article.
-
Sensing the neuronal glycocalyx by glial sialic acid binding immunoglobulin-like lectins.Neuroscience. 2014 Sep 5;275:113-24. doi: 10.1016/j.neuroscience.2014.05.061. Epub 2014 Jun 9. Neuroscience. 2014. PMID: 24924144 Review.
-
[Polysialic Acid for Immunomodulation in an Animal Model for Wet Age-Related Macular Degeneration (AMD)].Klin Monbl Augenheilkd. 2017 May;234(5):657-661. doi: 10.1055/s-0043-105138. Epub 2017 Apr 5. Klin Monbl Augenheilkd. 2017. PMID: 28380648 German.
-
Therapeutic potential to target sialylation and SIGLECs in neurodegenerative and psychiatric diseases.Front Neurol. 2024 Mar 11;15:1330874. doi: 10.3389/fneur.2024.1330874. eCollection 2024. Front Neurol. 2024. PMID: 38529039 Free PMC article. Review.
Cited by
-
PolySialic Acid Nanoparticles Actuate Complement-Factor-H-Mediated Inhibition of the Alternative Complement Pathway: A Safer Potential Therapy for Age-Related Macular Degeneration.Pharmaceuticals (Basel). 2024 Apr 17;17(4):517. doi: 10.3390/ph17040517. Pharmaceuticals (Basel). 2024. PMID: 38675477 Free PMC article.
-
Comprehensive Ocular and Systemic Safety Evaluation of Polysialic Acid-Decorated Immune Modulating Therapeutic Nanoparticles (PolySia-NPs) to Support Entry into First-in-Human Clinical Trials.Pharmaceuticals (Basel). 2024 Apr 9;17(4):481. doi: 10.3390/ph17040481. Pharmaceuticals (Basel). 2024. PMID: 38675441 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources


