A five-year observational prospective mono-center study of the efficacy of alemtuzumab in a real-world cohort of patients with multiple sclerosis
- PMID: 37808497
- PMCID: PMC10551138
- DOI: 10.3389/fneur.2023.1265354
A five-year observational prospective mono-center study of the efficacy of alemtuzumab in a real-world cohort of patients with multiple sclerosis
Abstract
Background: Alemtuzumab (ALZ) is a pulsed immune reconstitution therapy for multiple sclerosis (MS).
Objective: To assess basic characteristics, therapeutic effects, and prognostic biomarkers on clinical and imaging parameters of disease activity for relapsing-remitting MS (RRMS) patients selected for ALZ, in a real-world long-term setting.
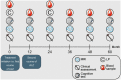
Methods: Fifty-one RRMS patients [female = 31; mean age 36 (standard deviation 7.1) years; median expanded disability status scale (EDSS) 2 (interquartile range (IQR) 1.5)] initiating ALZ treatment, were consecutively included. Patients were assessed at baseline and thereafter annually for 5 years with clinical measures, symbol digit modality test (SDMT), and magnetic resonance imaging (MRI). Concentrations of glial fibrillary acidic protein (GFAP), reflecting astrogliosis, and neurofilament light (NfL), reflecting axonal damage, were measured in cerebrospinal fluid (CSF) and serum samples collected at baseline and after 2 years in CSF, and annually in serum. Control subjects were symptomatic controls (SCs, n = 27), who were examined at baseline and after 5 years without evidence of neurological disease.
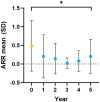
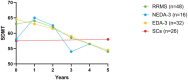
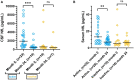
Results: While the mean annualized relapse rate was significantly reduced from baseline at each year of follow-up, disability was essentially maintained at a median EDSS of 1.5 and IQR between 1.13 and 2.25. New MRI activity was recorded in 26 patients (53%) over 5 years. The proportion of patients who achieved no evidence of disease activity (NEDA-3), 6-months confirmed disability worsening (CDW), and 6-months confirmed disability improvement (CDI) at 5 years were 33, 31, and 31%, respectively. The SDMT score was reduced for patients (p < 0.001), but unchanged for SCs. ALZ treatment did not change GFAP levels, whereas there was a significant decrease for RRMS patients in median CSF and serum NfL levels at follow-up [CSF month 24: 456 pg./mL (IQR 285.4) (p = 0.05); serum month 24: 6.7 pg/mL (IQR 4.7) (p < 0.01); serum month 60: 7.2 pg/mL (IQR 4.7) (p < 0.01)], compared to baseline [CSF: 1014 pg/mL (IQR 2832.5); serum 8.6 pg/mL (IQR 17.4)].
Conclusion: In this real-world mono-center population, we observed a progression-free survival of 69%, cumulative NEDA-3 of 33%, and reduced NfL levels, over a five-year follow-up. This confirms ALZ as an effective pulsed immune reconstitution therapy that significantly reduces neuro axonal loss, and therefore has the potential to reduce long-term neurological disability. ALZ did not appear to affect astrogliosis.
Keywords: alemtuzumab; glial fibrillary acidic protein; neurofilament light; prospective study; relapsing–remitting multiple sclerosis.
Copyright © 2023 Sandgren, Novakova, Nordin, Axelsson, Malmeström, Zetterberg and Lycke.
Conflict of interest statement
SS has received compensation for lectures and/or advisory board membership from Merck. LN has received lecture honoraria from Biogen, Novartis, Teva, Sanofi and has served on advisory boards for Merck, Janssen and Sanofi. MA has received compensation for lectures and/or advisory boards from Biogen, Genzyme, and Novartis. CM has received honoraria for lectures and advisory board memberships from Biogen, Merck, Novartis, and SanofiAventis. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB BBS, which is a part of the GU Ventures Incubator Program outside submitted work. JL has received travel support and/or lecture honoraria and has served on scientific advisory boards for Alexion, Almirall, Biogen, Bristol Myers Squibb, Celgene, Janssen, Merck, Novartis, Roche, and Sanofi; and has received unconditional research grants from Biogen and Novartis, and financial support from Sanofi for an investigator-initiated study. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


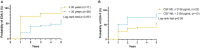
Similar articles
-
The effect of alemtuzumab on neurodegeneration in relapsing-remitting multiple sclerosis: A five-year prospective mono-center study.Mult Scler Relat Disord. 2024 Nov;91:105894. doi: 10.1016/j.msard.2024.105894. Epub 2024 Sep 13. Mult Scler Relat Disord. 2024. PMID: 39293124
-
Glial and neuroaxonal biomarkers in a multiple sclerosis (MS) cohort.Hell J Nucl Med. 2019 Sep-Dec;22 Suppl 2:113-121. Hell J Nucl Med. 2019. PMID: 31802051
-
Real-World Retrospective Analysis of Alemtuzumab Outcomes in Relapsing-Remitting Multiple Sclerosis: The LEMCAM Study.CNS Drugs. 2024 Mar;38(3):231-238. doi: 10.1007/s40263-024-01066-3. Epub 2024 Feb 28. CNS Drugs. 2024. PMID: 38418770 Free PMC article.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Alemtuzumab versus interferon beta 1a for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2017 Nov 27;11(11):CD010968. doi: 10.1002/14651858.CD010968.pub2. Cochrane Database Syst Rev. 2017. PMID: 29178444 Free PMC article. Review.
Cited by
-
The role of alemtuzumab in the development of secondary autoimmunity in multiple Sclerosis: a systematic review.J Neuroinflammation. 2024 Nov 1;21(1):281. doi: 10.1186/s12974-024-03263-9. J Neuroinflammation. 2024. PMID: 39487492 Free PMC article.
-
Effect of alemtuzumab over sNfL and sGFAP levels in multiple sclerosis.Front Immunol. 2024 Aug 19;15:1454474. doi: 10.3389/fimmu.2024.1454474. eCollection 2024. Front Immunol. 2024. PMID: 39224593 Free PMC article.
-
Real-World Study of Serum Neurofilament Light Chain Levels in Ocrelizumab-Treated People with Relapsing Multiple Sclerosis.J Pers Med. 2024 Jun 27;14(7):692. doi: 10.3390/jpm14070692. J Pers Med. 2024. PMID: 39063946 Free PMC article.
-
Association of serum glial fibrillary acidic protein with progression independent of relapse activity in multiple sclerosis.J Neurol. 2024 Jul;271(7):4412-4422. doi: 10.1007/s00415-024-12389-y. Epub 2024 Apr 26. J Neurol. 2024. PMID: 38668889 Free PMC article.
-
Serum neurofilament light for detecting disease activity in individual patients in multiple sclerosis: A 48-week prospective single-center study.Mult Scler. 2024 May;30(6):664-673. doi: 10.1177/13524585241237388. Epub 2024 Mar 13. Mult Scler. 2024. PMID: 38481083 Free PMC article.
References
-
- Jones JL, Phuah CL, Cox AL, Thompson SA, Ban M, Shawcross J, et al. . IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest. (2009) 119:2052–61. doi: 10.1172/JCI37878, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


