Effect of the Similarity of Formulations and Excipients of Approved Generic Drug Products on In Vivo Bioequivalence for Putative Biopharmaceutics Classification System Class III Drugs
- PMID: 37765334
- PMCID: PMC10534858
- DOI: 10.3390/pharmaceutics15092366
Effect of the Similarity of Formulations and Excipients of Approved Generic Drug Products on In Vivo Bioequivalence for Putative Biopharmaceutics Classification System Class III Drugs
Abstract
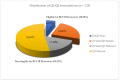
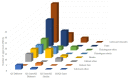
One of the potential essential factors that restricts generic industry from applying the Biopharmaceutics Classification System (BCS) Class III biowaiver is adherence to the stringent formulation criteria for formulation qualitative (Q1) sameness and quantitative (Q2) similarity. The present study has investigated formulations and excipients from 16 putative BCS Class III drug substances in a total of 19 drug products via 133 approved abbreviated new drug applications (ANDAs) containing in vivo bioequivalence (BE) studies in human subjects during the time period from 2006 to 2022. We included the BCS Class III drugs in this study by referring to published literature, the World Health Organization (WHO) BCS Class I-IV list, FDA internal assessments, and physicochemical properties (high solubility and low permeability) of specific drug substances. Based upon all 133 approved generic formulations in this study, the highest amount of each different compendial excipient with a total of 40 is defined as its corresponding typical amount that has not shown any potential impact on in vivo drug absorption. In the present study, although only 30.08% of the investigated generic formulations met Q1 the same/Q2 similar formulation criteria for the BCS Class III biowaiver, and while approximately 69.92% failed to meet those criteria with non-Q1/Q2 similar formulations, all test/reference ratios (T/R) and 90% confidence intervals for all instrumental PK parameters (AUC0-t, AUC0-inf, and Cmax) met the bioequivalence (BE) criteria (80-125%). The results of formulation assessment suggest that the commonly used excipients without atypical amounts did not impact absorption of 16 putative BCS Class III drug substances. The rate and extent of absorption of drugs appears to be more dependent upon the biopharmaceutic and physiochemical properties of BCS Class III drug substance and less, or not dependent upon their formulations, excipients, and the excipients class. Our findings may lead to a more flexible formulation design space regarding the stringent BCS Class III formulation criteria.
Keywords: Biopharmaceutics Classification System Class III; bioequivalence (BE); biowaiver; excipients; formulation; pharmacokinetic (PK); qualitatively (Q1)/quantitatively (Q2).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Feasibility of biowaiver extension to biopharmaceutics classification system class III drug products: cimetidine.Clin Pharmacokinet. 2006;45(4):385-99. doi: 10.2165/00003088-200645040-00004. Clin Pharmacokinet. 2006. PMID: 16584285 Clinical Trial.
-
Scientific considerations to move towards biowaiver for biopharmaceutical classification system class III drugs: How modeling and simulation can help.Biopharm Drug Dispos. 2021 Apr;42(4):118-127. doi: 10.1002/bdd.2274. Epub 2021 Apr 22. Biopharm Drug Dispos. 2021. PMID: 33759204 Review.
-
Bioequivalence Dissolution Test Criteria for Formulation Development of High Solubility-Low Permeability Drugs.Chem Pharm Bull (Tokyo). 2023;71(3):213-219. doi: 10.1248/cpb.c22-00685. Chem Pharm Bull (Tokyo). 2023. PMID: 36858526
-
Evaluation of Excipient Risk in BCS Class I and III Biowaivers.AAPS J. 2022 Jan 5;24(1):20. doi: 10.1208/s12248-021-00670-1. AAPS J. 2022. PMID: 34988701 Free PMC article. Review.
-
Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Metformin Hydrochloride.J Pharm Sci. 2021 Apr;110(4):1513-1526. doi: 10.1016/j.xphs.2021.01.011. Epub 2021 Jan 13. J Pharm Sci. 2021. PMID: 33450218
Cited by
-
A Method for the Colorimetric Quantification of Sodium Lauryl Sulphate in Tablets: A Proof of Concept.Pharmaceutics. 2024 Aug 21;16(8):1100. doi: 10.3390/pharmaceutics16081100. Pharmaceutics. 2024. PMID: 39204445 Free PMC article.
References
-
- U.S. Food and Drug Administration . The US FDA Guidance Documents, M9 Biopharmaceutics Classification System-Based Biowaivers. U.S. Food and Drug Administration; Silver Spring, MD, USA: 2021.
-
- Parr A., Hidalgo I.J., Bode C., Brown W., Yazdanian M., Gonzalez M.A., Sagawa K., Miller K., Jiang W., Stippler E.S. The Effect of Excipients on the Permeability of BCS Class III Compounds and Implications for Biowaivers. Pharm. Res. 2016;33:167–176. doi: 10.1007/s11095-015-1773-4. - DOI - PMC - PubMed
-
- Vaithianathan S., Haidar S.H., Zhang X., Jiang W., Avon C., Dowling T.C., Shao C., Kane M., Hoag S.W., Flasar M.H., et al. Effect of Common Excipients on the Oral Drug Absorption of Biopharmaceutics Classification System Class 3 Drugs Cimetidine and Acyclovir. J. Pharm. Sci. 2016;105:996–1005. doi: 10.1002/jps.24643. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


