The pyrenoid: the eukaryotic CO2-concentrating organelle
- PMID: 37279536
- PMCID: PMC10473226
- DOI: 10.1093/plcell/koad157
The pyrenoid: the eukaryotic CO2-concentrating organelle
Abstract
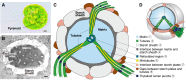
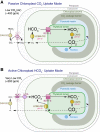
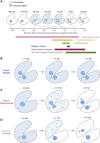
The pyrenoid is a phase-separated organelle that enhances photosynthetic carbon assimilation in most eukaryotic algae and the land plant hornwort lineage. Pyrenoids mediate approximately one-third of global CO2 fixation, and engineering a pyrenoid into C3 crops is predicted to boost CO2 uptake and increase yields. Pyrenoids enhance the activity of the CO2-fixing enzyme Rubisco by supplying it with concentrated CO2. All pyrenoids have a dense matrix of Rubisco associated with photosynthetic thylakoid membranes that are thought to supply concentrated CO2. Many pyrenoids are also surrounded by polysaccharide structures that may slow CO2 leakage. Phylogenetic analysis and pyrenoid morphological diversity support a convergent evolutionary origin for pyrenoids. Most of the molecular understanding of pyrenoids comes from the model green alga Chlamydomonas (Chlamydomonas reinhardtii). The Chlamydomonas pyrenoid exhibits multiple liquid-like behaviors, including internal mixing, division by fission, and dissolution and condensation in response to environmental cues and during the cell cycle. Pyrenoid assembly and function are induced by CO2 availability and light, and although transcriptional regulators have been identified, posttranslational regulation remains to be characterized. Here, we summarize the current knowledge of pyrenoid function, structure, components, and dynamic regulation in Chlamydomonas and extrapolate to pyrenoids in other species.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures


Similar articles
-
Pyrenoids: CO2-fixing phase separated liquid organelles.Biochim Biophys Acta Mol Cell Res. 2021 Apr;1868(5):118949. doi: 10.1016/j.bbamcr.2021.118949. Epub 2021 Jan 7. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33421532 Review.
-
A Rubisco-binding protein is required for normal pyrenoid number and starch sheath morphology in Chlamydomonas reinhardtii.Proc Natl Acad Sci U S A. 2019 Sep 10;116(37):18445-18454. doi: 10.1073/pnas.1904587116. Epub 2019 Aug 27. Proc Natl Acad Sci U S A. 2019. PMID: 31455733 Free PMC article.
-
The Eukaryotic CO2-Concentrating Organelle Is Liquid-like and Exhibits Dynamic Reorganization.Cell. 2017 Sep 21;171(1):148-162.e19. doi: 10.1016/j.cell.2017.08.008. Cell. 2017. PMID: 28938114 Free PMC article.
-
Pyrenoid proteomics reveals independent evolution of the CO2-concentrating organelle in chlorarachniophytes.Proc Natl Acad Sci U S A. 2024 Mar 5;121(10):e2318542121. doi: 10.1073/pnas.2318542121. Epub 2024 Feb 26. Proc Natl Acad Sci U S A. 2024. PMID: 38408230 Free PMC article.
-
Pyrenoid: Organelle with efficient CO2-Concentrating mechanism in algae.J Plant Physiol. 2023 Aug;287:154044. doi: 10.1016/j.jplph.2023.154044. Epub 2023 Jun 25. J Plant Physiol. 2023. PMID: 37392525 Review.
Cited by
-
Algal pyrenoid protein can condense plant Rubiscos: a step towards boosting carbon fixation in crops.Nat Plants. 2024 Nov;10(11):1625-1626. doi: 10.1038/s41477-024-01813-w. Nat Plants. 2024. PMID: 39384946 No abstract available.
-
Perspectives on improving photosynthesis to increase crop yield.Plant Cell. 2024 Oct 3;36(10):3944-3973. doi: 10.1093/plcell/koae132. Plant Cell. 2024. PMID: 38701340 Free PMC article. Review.
-
Structure, biogenesis, and evolution of thylakoid membranes.Plant Cell. 2024 Oct 3;36(10):4014-4035. doi: 10.1093/plcell/koae102. Plant Cell. 2024. PMID: 38567528 Review.
-
Algal chloroplast pyrenoids: Evidence for convergent evolution.Proc Natl Acad Sci U S A. 2024 Apr 2;121(14):e2402546121. doi: 10.1073/pnas.2402546121. Epub 2024 Mar 21. Proc Natl Acad Sci U S A. 2024. PMID: 38513078 Free PMC article. No abstract available.
-
Chloroplast ATP synthase: From structure to engineering.Plant Cell. 2024 Oct 3;36(10):3974-3996. doi: 10.1093/plcell/koae081. Plant Cell. 2024. PMID: 38484126 Review.
References
-
- Atkinson N, Velanis CN, Wunder T, Clarke DJ, Mueller-Cajar O, McCormick AJ. The pyrenoidal linker protein EPYC1 phase separates with hybrid Arabidopsis-Chlamydomonas Rubisco through interactions with the algal Rubisco small subunit. J Exp Bot. 2019:70(19):5271–5285. 10.1093/jxb/erz275 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


