Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice
- PMID: 37259325
- PMCID: PMC9967428
- DOI: 10.3390/ph16020175
Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice
Abstract
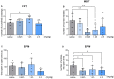
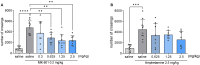
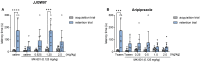
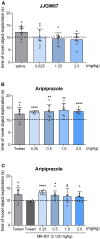
Depression, anxiety, and schizophrenia may coexist in psychiatric patients. Moreover, these disorders are very often associated with cognitive impairments. However, pharmacotherapy of these conditions remains challenging due to limited drug effectiveness or numerous side effects. Therefore, there is an urgent need to develop novel multimodal compounds that can be used to treat depression, anxiety, and schizophrenia, as well as memory deficits. Thus, this study aimed to evaluate the potential antidepressant-like, anxiolytic-like, antipsychotic-like effects, and anti-amnesic properties, of the novel arylpiperazine derivative of salicylamide, JJGW07, with an affinity towards serotonin 5-HT1A, 5-HT2A, and 5-HT7 and dopamine D2 receptors. Firstly, we investigated the compound's affinity for 5-HT6 receptors and its functional activity by using in vitro assays. JJGW07 did not bind to 5-HT6 receptors and showed antagonistic properties for 5-HT1A, 5-HT2A, 5-HT7, and D2 receptors. Based on the receptor profile, we performed behavioral studies in mice to evaluate the antidepressant-like, anxiolytic-like, and antipsychotic-like activity of the tested compound using forced swim and tail suspension tests; four-plate, marble-burying, and elevated plus maze tests; and MK-801- and amphetamine-induced hyperlocomotion tests, respectively. JJGW07 revealed antidepressant-like properties in the tail suspension test, anxiolytic-like effects in the four-plate and marble-burying tests, and antipsychotic-like activity in the MK-801-induced hyperlocomotion test. Importantly, the tested compound did not induce catalepsy and motor impairments or influence locomotor activity in rodents. Finally, to assess the potential procognitive and anti-amnesic properties of JJGW07, we used passive avoidance and object recognition tests in mice. JJGW07 demonstrated positive effects on long-term emotional memory and also ameliorated MK-801-induced emotional memory impairments in mice, but showed no procognitive properties in the case of recognition memory. Our results encourage the search for new compounds among salicylamide derivatives, which could be model structures with multitarget mechanisms of action that could be used in psychiatric disorder therapy.
Keywords: anti-amnesic effect; antidepressant-like activity; antipsychotic-like properties; anxiolytic-like action; memory-enhancing; serotonin receptor antagonist.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

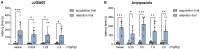
Similar articles
-
Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents.Int J Mol Sci. 2022 Dec 14;23(24):15929. doi: 10.3390/ijms232415929. Int J Mol Sci. 2022. PMID: 36555568 Free PMC article.
-
Potential Anti-Amnesic Activity of a Novel Multimodal Derivative of Salicylamide, JJGW08, in Mice.Pharmaceuticals (Basel). 2023 Mar 6;16(3):399. doi: 10.3390/ph16030399. Pharmaceuticals (Basel). 2023. PMID: 36986498 Free PMC article.
-
HBK-7 - A new xanthone derivative and a 5-HT1A receptor antagonist with antidepressant-like properties.Pharmacol Biochem Behav. 2016 Jul-Aug;146-147:35-43. doi: 10.1016/j.pbb.2016.04.005. Epub 2016 Apr 27. Pharmacol Biochem Behav. 2016. PMID: 27132236
-
Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research.CNS Drugs. 2013 Sep;27(9):703-16. doi: 10.1007/s40263-013-0071-0. CNS Drugs. 2013. PMID: 23757185 Review.
-
[Pharmacological properties of vortioxetine and its pre-clinical consequences].Encephale. 2016 Feb;42(1 Suppl 1):1S12-23. doi: 10.1016/S0013-7006(16)30015-X. Encephale. 2016. PMID: 26879252 Review. French.
Cited by
-
Formulation, Characterization, and Evaluation of β-Cyclodextrin Functionalized Hypericin Loaded Nanocarriers.ACS Omega. 2023 Oct 3;8(41):38191-38203. doi: 10.1021/acsomega.3c04444. eCollection 2023 Oct 17. ACS Omega. 2023. PMID: 37867680 Free PMC article.
References
-
- World Health Organisation Mental Disorders. [(accessed on 22 December 2022)]; Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders.
-
- Gémes K., Bergström J., Papola D., Barbui C., Lam A.I.F., Hall B.J., Seedat S., Morina N., Quero S., Campos D., et al. Symptoms of anxiety and depression during the COVID-19 pandemic in six European countries and Australia—Differences by prior mental disorders and migration status. J. Affect. Disord. 2022;311:214–223. doi: 10.1016/j.jad.2022.05.082. - DOI - PMC - PubMed
-
- Jaśkowska J., Drabczyk A.K., Śliwa P., Jodłowski P., Pindelska E., Kułaga D., Zaręba P., Majka Z., Siwek A., Wolak M., et al. Ultrasound assisted one-pot synthesis and preliminary in vitro studies of salicylamide arylpiperazines as dual 5-HT1A/5-HT7 ligands. J. Mol. Struct. 2022;1275:134585. doi: 10.1016/j.molstruc.2022.134585. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources


