Structures of human primosome elongation complexes
- PMID: 37069376
- PMCID: PMC10268227
- DOI: 10.1038/s41594-023-00971-3
Structures of human primosome elongation complexes
Abstract
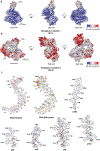
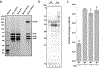
The synthesis of RNA-DNA primer by primosome requires coordination between primase and DNA polymerase α subunits, which is accompanied by unknown architectural rearrangements of multiple domains. Using cryogenic electron microscopy, we solved a 3.6 Å human primosome structure caught at an early stage of RNA primer elongation with deoxynucleotides. The structure confirms a long-standing role of primase large subunit and reveals new insights into how primosome is limited to synthesizing short RNA-DNA primers.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

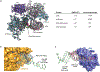




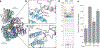
Similar articles
-
Primase and polymerase α tango to make an RNA-DNA hybrid primer.FEBS J. 2024 May;291(9):1889-1891. doi: 10.1111/febs.17129. Epub 2024 Apr 5. FEBS J. 2024. PMID: 38581152
-
CryoEM insights into RNA primer synthesis by the human primosome.FEBS J. 2024 Apr;291(8):1813-1829. doi: 10.1111/febs.17082. Epub 2024 Feb 9. FEBS J. 2024. PMID: 38335062
-
Mechanism of Concerted RNA-DNA Primer Synthesis by the Human Primosome.J Biol Chem. 2016 May 6;291(19):10006-20. doi: 10.1074/jbc.M116.717405. Epub 2016 Mar 14. J Biol Chem. 2016. PMID: 26975377 Free PMC article.
-
Elaborated Action of the Human Primosome.Genes (Basel). 2017 Feb 8;8(2):62. doi: 10.3390/genes8020062. Genes (Basel). 2017. PMID: 28208743 Free PMC article. Review.
-
The Pol α-primase complex.Subcell Biochem. 2012;62:157-69. doi: 10.1007/978-94-007-4572-8_9. Subcell Biochem. 2012. PMID: 22918585 Review.
Cited by
-
Small LEA proteins mitigate air-water interface damage to fragile cryo-EM samples during plunge freezing.Nat Commun. 2024 Sep 4;15(1):7705. doi: 10.1038/s41467-024-52091-1. Nat Commun. 2024. PMID: 39231985 Free PMC article.
-
Essential and multifunctional mpox virus E5 helicase-primase in double and single hexamer.Sci Adv. 2024 Aug 23;10(34):eadl1150. doi: 10.1126/sciadv.adl1150. Epub 2024 Aug 21. Sci Adv. 2024. PMID: 39167653 Free PMC article.
-
Telomere C-Strand Fill-In Machinery: New Insights into the Human CST-DNA Polymerase Alpha-Primase Structures and Functions.Subcell Biochem. 2024;104:73-100. doi: 10.1007/978-3-031-58843-3_5. Subcell Biochem. 2024. PMID: 38963484 Review.
-
Primase and polymerase α tango to make an RNA-DNA hybrid primer.FEBS J. 2024 May;291(9):1889-1891. doi: 10.1111/febs.17129. Epub 2024 Apr 5. FEBS J. 2024. PMID: 38581152
-
Starting DNA Synthesis: Initiation Processes during the Replication of Chromosomal DNA in Humans.Genes (Basel). 2024 Mar 14;15(3):360. doi: 10.3390/genes15030360. Genes (Basel). 2024. PMID: 38540419 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


