Glia-Neurotrophic Factor Relationships: Possible Role in Pathobiology of Neuroinflammation-Related Brain Disorders
- PMID: 37047292
- PMCID: PMC10094105
- DOI: 10.3390/ijms24076321
Glia-Neurotrophic Factor Relationships: Possible Role in Pathobiology of Neuroinflammation-Related Brain Disorders
Abstract
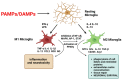
Neurotrophic factors (NTFs) play an important role in maintaining homeostasis of the central nervous system (CNS) by regulating the survival, differentiation, maturation, and development of neurons and by participating in the regeneration of damaged tissues. Disturbances in the level and functioning of NTFs can lead to many diseases of the nervous system, including degenerative diseases, mental diseases, and neurodevelopmental disorders. Each CNS disease is characterized by a unique pathomechanism, however, the involvement of certain processes in its etiology is common, such as neuroinflammation, dysregulation of NTFs levels, or mitochondrial dysfunction. It has been shown that NTFs can control the activation of glial cells by directing them toward a neuroprotective and anti-inflammatory phenotype and activating signaling pathways responsible for neuronal survival. In this review, our goal is to outline the current state of knowledge about the processes affected by NTFs, the crosstalk between NTFs, mitochondria, and the nervous and immune systems, leading to the inhibition of neuroinflammation and oxidative stress, and thus the inhibition of the development and progression of CNS disorders.
Keywords: glial cells; mitochondrial dysfunction; neuroinflammation; neuroprotection; neurotrophic factors; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Intranasal administration: a potential solution for cross-BBB delivering neurotrophic factors.Histol Histopathol. 2012 May;27(5):537-48. doi: 10.14670/HH-27.537. Histol Histopathol. 2012. PMID: 22419018 Review.
-
Cardiolipin in Central Nervous System Physiology and Pathology.Cell Mol Neurobiol. 2017 Oct;37(7):1161-1172. doi: 10.1007/s10571-016-0458-9. Epub 2016 Dec 30. Cell Mol Neurobiol. 2017. PMID: 28039536 Review.
-
Transfection of C6 glioma cells with glia maturation factor upregulates brain-derived neurotrophic factor and nerve growth factor: trophic effects and protection against ethanol toxicity in cerebellar granule cells.Brain Res. 2000 May 19;865(1):59-76. doi: 10.1016/s0006-8993(00)02194-6. Brain Res. 2000. PMID: 10814733
-
The mechanism of memory impairment induced by Aβ chronic administration involves imbalance between cytokines and neurotrophins in the rat hippocampus.Curr Alzheimer Res. 2011 Jun;8(4):410-20. doi: 10.2174/156720511795745366. Curr Alzheimer Res. 2011. PMID: 21244354
-
Neurotrophic Factors in Parkinson's Disease: Clinical Trials, Open Challenges and Nanoparticle-Mediated Delivery to the Brain.Front Cell Neurosci. 2021 Jun 2;15:682597. doi: 10.3389/fncel.2021.682597. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34149364 Free PMC article. Review.
Cited by
-
Autologous Fat Grafting-A Panacea for Scar Tissue Therapy?Cells. 2024 Aug 20;13(16):1384. doi: 10.3390/cells13161384. Cells. 2024. PMID: 39195271 Free PMC article. Review.
-
Leukotriene signaling in neurodegeneration: implications for treatment strategies.Inflammopharmacology. 2024 Dec;32(6):3571-3584. doi: 10.1007/s10787-024-01557-1. Epub 2024 Aug 21. Inflammopharmacology. 2024. PMID: 39167313 Review.
-
Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction.Brain Sci. 2024 May 30;14(6):558. doi: 10.3390/brainsci14060558. Brain Sci. 2024. PMID: 38928557 Free PMC article. Review.
-
Altered blood parameters in "major depression" patients receiving repetitive transcranial magnetic stimulation (rTMS) therapy: a randomized case-control study.Transl Psychiatry. 2024 Jun 25;14(1):264. doi: 10.1038/s41398-024-02942-8. Transl Psychiatry. 2024. PMID: 38918365 Free PMC article. Clinical Trial.
-
Immunological dimensions of neuroinflammation and microglial activation: exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression.Front Immunol. 2024 Jan 8;14:1305933. doi: 10.3389/fimmu.2023.1305933. eCollection 2023. Front Immunol. 2024. PMID: 38259497 Free PMC article. Review.
References
-
- Takeuchi H. Roles of Glial Cells in Neuroinflammation and Neurodegeneration. Clin. Exp. Neuroimmunol. 2013;4:2–16. doi: 10.1111/cen3.12059. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


