Pharmacotherapy of obesity: an update on the available medications and drugs under investigation
- PMID: 36992862
- PMCID: PMC10041469
- DOI: 10.1016/j.eclinm.2023.101882
Pharmacotherapy of obesity: an update on the available medications and drugs under investigation
Abstract
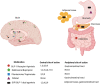
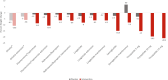
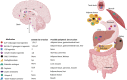
Obesity is an epidemic and a public health threat. Medical weight management remains one of the options for the treatment of excess weight and recent advances have revolutionized how we treat, and more importantly how we will be treating obesity in the near future. Metreleptin and Setmelanotide are currently indicated for rare obesity syndromes, and 5 other medications (orlistat, phentermine/topiramate, naltrexone/bupropion, liraglutide, semaglutide) are approved for non-syndromic obesity. Tirzepatide is about to be approved, and other drugs, with exciting novel mechanisms of action primarily based on incretins, are currently being investigated in different phases of clinical trials. The majority of these compounds act centrally, to reduce appetite and increase satiety, and secondarily, in the gastrointestinal tract to slow gastric emptying. All anti-obesity medications improve weight and metabolic parameters, with variable potency and effects depending on the specific drug. The currently available data do not support a reduction in hard cardiovascular outcomes, but it is almost certain that such data are forthcoming in the very near future. The choice of the anti-obesity medication needs to take into consideration the patient's clinical and biochemical profile, co-morbidities, and drug contra-indications, as well as expected degree of weight loss and improvements in cardio-renal and metabolic risk. It also remains to be seen whether precision medicine may offer personalized solutions to individuals with obesity, and whether it may represent the future of medical weight management along with the development of novel, very potent, anti-obesity medications currently in the pipeline.
Funding: None.
Keywords: Anti-obesity medications; Obesity; Weight management.
© 2023 The Authors.
Conflict of interest statement
C.S.M. has been a shareholder of and reports grants through his institution from Merck, grants through his Institution and personal consulting fees from Coherus Inc. and AltrixBio, he reports grants through his institution and personal consulting fees from Novo Nordisk, reports personal consulting fees and support with research reagents from Ansh Inc., reports personal consulting fees from Genfit, Lumos, Amgen, Corcept, Intercept, Astra Zeneca, 89bio and Regeneron, reports support (educational activity meals at and through his institution) from Amarin, Novo Nordisk and travel support and fees from TMIOA, Elsevier, the California Walnut Commission, College Internationale Researche Servier and the Cardio Metabolic Health Conference. None is related to the work presented herein. All other authors declare no conflict of interest.
Figures
Similar articles
-
Drugs for Treating Obesity.Handb Exp Pharmacol. 2022;274:387-414. doi: 10.1007/164_2021_560. Handb Exp Pharmacol. 2022. PMID: 34783910
-
Review article: Pharmacologic management of obesity - updates on approved medications, indications and risks.Aliment Pharmacol Ther. 2024 Feb;59(4):475-491. doi: 10.1111/apt.17856. Epub 2024 Jan 2. Aliment Pharmacol Ther. 2024. PMID: 38169126 Review.
-
Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
-
Pharmacotherapy of obesity: Available medications and drugs under investigation.Metabolism. 2019 Mar;92:170-192. doi: 10.1016/j.metabol.2018.10.010. Epub 2018 Nov 1. Metabolism. 2019. PMID: 30391259 Review.
-
Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022.Obes Pillars. 2022 Apr 15;2:100018. doi: 10.1016/j.obpill.2022.100018. eCollection 2022 Jun. Obes Pillars. 2022. PMID: 37990711 Free PMC article.
Cited by
-
Comparative Effectiveness of Bariatric Metabolic Surgery Versus Glucagon-Like Peptide-1 Receptor Agonists on Cardiovascular Outcomes and Mortality: A Meta-Analysis.Cureus. 2024 Oct 17;16(10):e71684. doi: 10.7759/cureus.71684. eCollection 2024 Oct. Cureus. 2024. PMID: 39552962 Free PMC article. Review.
-
Integrating Genetic Insights, Technological Advancements, Screening, and Personalized Pharmacological Interventions in Childhood Obesity.Adv Ther. 2024 Nov 13. doi: 10.1007/s12325-024-03057-8. Online ahead of print. Adv Ther. 2024. PMID: 39535684 Review.
-
A systematic review of obesity burden in Saudi Arabia: Prevalence and associated co-morbidities.Saudi Pharm J. 2024 Nov;32(11):102192. doi: 10.1016/j.jsps.2024.102192. Epub 2024 Oct 24. Saudi Pharm J. 2024. PMID: 39525490 Free PMC article. Review.
-
Effect of survodutide, a glucagon and GLP-1 receptor dual agonist, on weight loss: a meta-analysis of randomized controlled trials.Diabetol Metab Syndr. 2024 Nov 6;16(1):264. doi: 10.1186/s13098-024-01501-x. Diabetol Metab Syndr. 2024. PMID: 39508238 Free PMC article.
-
The Therapeutic Potential of Glucagon-like Peptide 1 Receptor Agonists in Traumatic Brain Injury.Pharmaceuticals (Basel). 2024 Oct 1;17(10):1313. doi: 10.3390/ph17101313. Pharmaceuticals (Basel). 2024. PMID: 39458954 Free PMC article. Review.
References
-
- Tiwari A., Balasundaram P. StatPearls Publishing; 2021. Public health considerations regarding obesity. - PubMed
-
- Ritchie H., Roser M. Obesity. 2017. https://ourworldindata.org/obesity
Publication types
LinkOut - more resources
Full Text Sources


