Potential Anti-Amnesic Activity of a Novel Multimodal Derivative of Salicylamide, JJGW08, in Mice
- PMID: 36986498
- PMCID: PMC10056859
- DOI: 10.3390/ph16030399
Potential Anti-Amnesic Activity of a Novel Multimodal Derivative of Salicylamide, JJGW08, in Mice
Abstract
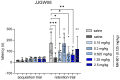
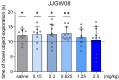
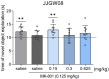
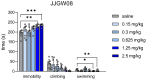
Memory impairments constitute a significant problem worldwide, and the COVID-19 pandemic dramatically increased the prevalence of cognitive deficits. Patients with cognitive deficits, specifically memory disturbances, have underlying comorbid conditions such as schizophrenia, anxiety, or depression. Moreover, the available treatment options have unsatisfactory effectiveness. Therefore, there is a need to search for novel procognitive and anti-amnesic drugs with additional pharmacological activity. One of the important therapeutic targets involved in the modulation of learning and memory processes are serotonin receptors, including 5-HT1A, 5-HT6, and 5-HT7, which also play a role in the pathophysiology of depression. Therefore, this study aimed to assess the anti-amnesic and antidepressant-like potential of JJGW08, a novel arylpiperazine alkyl derivative of salicylamide with strong antagonistic properties at 5-HT1A and D2 receptors and weak at 5-HT2A and 5-HT7 receptors in rodents. First, we investigated the compound's affinity for 5-HT6 receptors using the radioligand assays. Next, we assessed the influence of the compound on long-term emotional and recognition memory. Further, we evaluated whether the compound could protect against MK-801-induced cognitive impairments. Finally, we determined the potential antidepressant-like activity of the tested compound. We found that JJGW08 possessed no affinity for 5-HT6 receptors. Furthermore, JJGW08 protected mice against MK-801-induced recognition and emotional memory deficits but showed no antidepressant-like effects in rodents. Therefore, our preliminary study may suggest that blocking serotonin receptors, especially 5-HT1A and 5-HT7, might be beneficial in treating cognitive impairments, but it requires further investigation.
Keywords: anti-amnesic effect; antidepressant-like activity; cognition; long-term memory; serotonin receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice.Pharmaceuticals (Basel). 2023 Jan 24;16(2):175. doi: 10.3390/ph16020175. Pharmaceuticals (Basel). 2023. PMID: 37259325 Free PMC article.
-
Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents.Int J Mol Sci. 2022 Dec 14;23(24):15929. doi: 10.3390/ijms232415929. Int J Mol Sci. 2022. PMID: 36555568 Free PMC article.
-
New 8-aminoalkyl derivatives of purine-2,6-dione with arylalkyl, allyl or propynyl substituents in position 7, their 5-HT1A, 5-HT2A, and 5-HT7 receptor affinity and pharmacological evaluation.Pharmacol Rep. 2013;65(1):15-29. doi: 10.1016/s1734-1140(13)70960-5. Pharmacol Rep. 2013. PMID: 23563020
-
Memory formation and memory alterations: 5-HT6 and 5-HT7 receptors, novel alternative.Rev Neurosci. 2014;25(3):325-56. doi: 10.1515/revneuro-2014-0001. Rev Neurosci. 2014. PMID: 24698823 Review.
-
[Pharmacological properties of vortioxetine and its pre-clinical consequences].Encephale. 2016 Feb;42(1 Suppl 1):1S12-23. doi: 10.1016/S0013-7006(16)30015-X. Encephale. 2016. PMID: 26879252 Review. French.
References
-
- World Health Organization Dementia. [(accessed on 26 October 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia.
-
- Keijsers K., Broeders M., Baptista Lopes V., Klinkert A., van Baar J., Nahar-van Venrooij L., Kerckhoffs A. Memory impairment and concentration problems in COVID-19 survivors 8 weeks after non-ICU hospitalization: A retrospective cohort study. J. Med. Virol. 2022;94:4512–4517. doi: 10.1002/jmv.27831. - DOI - PMC - PubMed
-
- Crivelli L., Palmer K., Calandri I., Guekht A., Beghi E., Carroll W., Frontera J., García-Azorín D., Westenberg E., Winkler A.S., et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers. Dement. 2022;18:1047–1066. doi: 10.1002/alz.12644. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources


