Progress towards a glycoconjugate vaccine against Group A Streptococcus
- PMID: 36977677
- PMCID: PMC10043865
- DOI: 10.1038/s41541-023-00639-5
Progress towards a glycoconjugate vaccine against Group A Streptococcus
Abstract
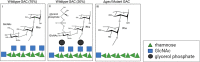
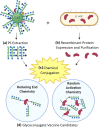
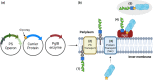
The Group A Carbohydrate (GAC) is a defining feature of Group A Streptococcus (Strep A) or Streptococcus pyogenes. It is a conserved and simple polysaccharide, comprising a rhamnose backbone and GlcNAc side chains, further decorated with glycerol phosphate on approximately 40% GlcNAc residues. Its conservation, surface exposure and antigenicity have made it an interesting focus on Strep A vaccine design. Glycoconjugates containing this conserved carbohydrate should be a key approach towards the successful mission to build a universal Strep A vaccine candidate. In this review, a brief introduction to GAC, the main carbohydrate component of Strep A bacteria, and a variety of published carrier proteins and conjugation technologies are discussed. Components and technologies should be chosen carefully for building affordable Strep A vaccine candidates, particularly for low- and middle-income countries (LMICs). Towards this, novel technologies are discussed, such as the prospective use of bioconjugation with PglB for rhamnose polymer conjugation and generalised modules for membrane antigens (GMMA), particularly as low-cost solutions to vaccine production. Rational design of "double-hit" conjugates encompassing species specific glycan and protein components would be beneficial and production of a conserved vaccine to target Strep A colonisation without invoking an autoimmune response would be ideal.
© 2023. Crown.
Conflict of interest statement
H.C.D. holds a patent on the rhamnose polysaccharide platform technology (WO2020249737A1). B.W.W. holds a patent for an
Figures

Similar articles
-
GMMA as an Alternative Carrier for a Glycoconjugate Vaccine against Group A Streptococcus.Vaccines (Basel). 2022 Jun 28;10(7):1034. doi: 10.3390/vaccines10071034. Vaccines (Basel). 2022. PMID: 35891202 Free PMC article.
-
Elucidating the role of N-acetylglucosamine in Group A Carbohydrate for the development of an effective glycoconjugate vaccine against Group A Streptococcus.Carbohydr Polym. 2023 Jul 1;311:120736. doi: 10.1016/j.carbpol.2023.120736. Epub 2023 Feb 23. Carbohydr Polym. 2023. PMID: 37028871
-
The molecular mechanism of N-acetylglucosamine side-chain attachment to the Lancefield group A carbohydrate in Streptococcus pyogenes.J Biol Chem. 2017 Nov 24;292(47):19441-19457. doi: 10.1074/jbc.M117.815910. Epub 2017 Oct 11. J Biol Chem. 2017. PMID: 29021255 Free PMC article.
-
Hijacking bacterial glycosylation for the production of glycoconjugates, from vaccines to humanised glycoproteins.J Pharm Pharmacol. 2015 Mar;67(3):338-50. doi: 10.1111/jphp.12321. Epub 2014 Sep 22. J Pharm Pharmacol. 2015. PMID: 25244672 Free PMC article. Review.
-
Improving efficacy of glycoconjugate vaccines: from chemical conjugates to next generation constructs.Curr Opin Immunol. 2020 Aug;65:42-49. doi: 10.1016/j.coi.2020.03.015. Epub 2020 Apr 30. Curr Opin Immunol. 2020. PMID: 32361591 Review.
Cited by
-
Invasive group A Streptococcus infection (Streptococcus pyogenes): Current situation in Spain.Rev Esp Quimioter. 2024 Dec;37(6):454-471. doi: 10.37201/req/067.2024. Epub 2024 Jul 30. Rev Esp Quimioter. 2024. PMID: 39076142 Free PMC article. Review.
-
Strep A: challenges, opportunities, vaccine-based solutions, and economics.NPJ Vaccines. 2024 Apr 19;9(1):80. doi: 10.1038/s41541-024-00863-7. NPJ Vaccines. 2024. PMID: 38641634 Free PMC article. Review.
-
Immunomodulating Enzymes from Streptococcus pyogenes-In Pathogenesis, as Biotechnological Tools, and as Biological Drugs.Microorganisms. 2024 Jan 18;12(1):200. doi: 10.3390/microorganisms12010200. Microorganisms. 2024. PMID: 38258026 Free PMC article. Review.
-
Research opportunities for the primordial prevention of rheumatic fever and rheumatic heart disease-streptococcal vaccine development: a national heart, lung and blood institute workshop report.BMJ Glob Health. 2023 Dec 12;8(Suppl 9):e013534. doi: 10.1136/bmjgh-2023-013534. BMJ Glob Health. 2023. PMID: 38164699 Free PMC article. Review.
-
Streptococcus pyogenes: Pathogenesis and the Current Status of Vaccines.Vaccines (Basel). 2023 Sep 21;11(9):1510. doi: 10.3390/vaccines11091510. Vaccines (Basel). 2023. PMID: 37766186 Free PMC article. Review.
References
-
- WHO. The Current Evidence for the Burden of Group A Streptococcal Diseases. https://apps.who.int/iris/handle/10665/69063 (2005).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources


