A Novel Antithrombocytopenia Agent, Rhizoma cibotii, Promotes Megakaryopoiesis and Thrombopoiesis through the PI3K/AKT, MEK/ERK, and JAK2/STAT3 Signaling Pathways
- PMID: 36430539
- PMCID: PMC9694118
- DOI: 10.3390/ijms232214060
A Novel Antithrombocytopenia Agent, Rhizoma cibotii, Promotes Megakaryopoiesis and Thrombopoiesis through the PI3K/AKT, MEK/ERK, and JAK2/STAT3 Signaling Pathways
Abstract
Background: Cibotii rhizoma (CR) is a famous traditional Chinese medicine (TCM) used to treat bleeding, rheumatism, lumbago, etc. However, its therapeutic effects and mechanism against thrombocytopenia are still unknown so far. In the study, we investigated the effects of aqueous extracts of Cibotii rhizoma (AECRs) against thrombocytopenia and its molecular mechanism.
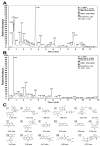
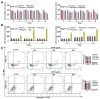
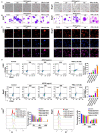
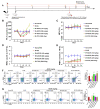
Methods: Giemsa staining, phalloidin staining, and flow cytometry were performed to measure the effect of AECRs on the megakaryocyte differentiation in K562 and Meg-01 cells. A radiation-induced thrombocytopenia mouse model was constructed to assess the therapeutic actions of AECRs on thrombocytopenia. Network pharmacology and experimental verification were carried out to clarify its mechanism against thrombocytopenia.
Results: AECRs promoted megakaryocyte differentiation in K562 and Meg-01 cells and accelerated platelet recovery and megakaryopoiesis with no systemic toxicity in radiation-induced thrombocytopenia mice. The PI3K/AKT, MEK/ERK, and JAK2/STAT3 signaling pathways contributed to AECR-induced megakaryocyte differentiation. The suppression of the above signaling pathways by their inhibitors blocked AERC-induced megakaryocyte differentiation.
Conclusions: AECRs can promote megakaryopoiesis and thrombopoiesis through activating PI3K/AKT, MEK/ERK, and JAK2/STAT3 signaling pathways, which has the potential to treat radiation-induced thrombocytopenia in the clinic.
Keywords: Cibotii rhizoma; JAK2/STAT3; MEK/ERK; PI3K/AKT; megakaryocyte differentiation; network pharmacology; thrombocytopenia.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
The combination of machine learning and transcriptomics reveals a novel megakaryopoiesis inducer, MO-A, that promotes thrombopoiesis by activating FGF1/FGFR1/PI3K/Akt/NF-κB signaling.Eur J Pharmacol. 2023 Apr 5;944:175604. doi: 10.1016/j.ejphar.2023.175604. Epub 2023 Feb 18. Eur J Pharmacol. 2023. PMID: 36804544
-
Hirsutine, a novel megakaryopoiesis inducer, promotes thrombopoiesis via MEK/ERK/FOG1/TAL1 signaling.Phytomedicine. 2022 Jul 20;102:154150. doi: 10.1016/j.phymed.2022.154150. Epub 2022 May 5. Phytomedicine. 2022. PMID: 35569185
-
Xanthotoxin, a novel inducer of platelet formation, promotes thrombocytopoiesis via IL-1R1 and MEK/ERK signaling.Biomed Pharmacother. 2023 Jul;163:114811. doi: 10.1016/j.biopha.2023.114811. Epub 2023 May 8. Biomed Pharmacother. 2023. PMID: 37156117
-
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health.Oncotarget. 2011 Mar;2(3):135-64. doi: 10.18632/oncotarget.240. Oncotarget. 2011. PMID: 21411864 Free PMC article. Review.
-
Megakaryocyte modification of platelets in thrombocytopenia.Curr Opin Hematol. 2018 Sep;25(5):410-415. doi: 10.1097/MOH.0000000000000451. Curr Opin Hematol. 2018. PMID: 29985173 Review.
Cited by
-
Suppressing inflammatory signals and apoptosis-linked sphingolipid metabolism underlies therapeutic potential of Qing-Jin-Hua-Tan decoction against chronic obstructive pulmonary disease.Heliyon. 2024 Jan 18;10(3):e24336. doi: 10.1016/j.heliyon.2024.e24336. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38318072 Free PMC article.
-
Mass Spectrometric Identification of Metabolites after Magnetic-Pulse Treatment of Infected Pyrus communis L. Microplants.Int J Mol Sci. 2023 Nov 26;24(23):16776. doi: 10.3390/ijms242316776. Int J Mol Sci. 2023. PMID: 38069098 Free PMC article.
References
-
- Praveen Kumar M.K., Shyama S.K., Sonaye B.S., Naik U.R., Kadam S.B., Bipin P.D., D’Costa A., Chaubey R.C. Evaluation of γ-radiation-induced DNA damage in two species of bivalves and their relative sensitivity using comet assay. Aquat. Toxicol. 2014;150:1–8. doi: 10.1016/j.aquatox.2014.02.007. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


