Development of a salivary autoantibody biomarker panel for diagnosis of oral cavity squamous cell carcinoma
- PMID: 36387116
- PMCID: PMC9659860
- DOI: 10.3389/fonc.2022.968570
Development of a salivary autoantibody biomarker panel for diagnosis of oral cavity squamous cell carcinoma
Abstract
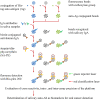
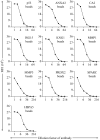
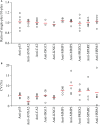
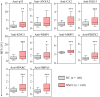
Oral cavity squamous cell carcinoma (OSCC) is a destructive disease with increasing incidence. OSCC is usually diagnosed at an advanced stage, which leads to poor outcomes of OSCC patients. Currently, there is a lack of biomarkers with sufficient effectiveness in early diagnosis of OSCC. To ameliorate OSCC screening, we evaluated the performances of salivary autoantibodies (auto-Abs) to nine proteins (ANXA2, CA2, ISG15, KNG1, MMP1, MMP3, PRDX2, SPARC, and HSPA5) as OSCC biomarkers. A multiplexed immunoassay using a fluorescence bead-based suspension array system was established for simultaneous assessment of the salivary levels of the above nine auto-Abs and a known OSCC-associated auto-Ab, anti-p53. Compared to healthy individuals (n = 140), the salivary levels of nine auto-Abs were significantly elevated in OSCC patients (n = 160). Notably, the salivary levels of the 10 auto-Abs in the early-stage OSCC patients (n = 102) were higher than that in the healthy group. Most importantly, utilizing a marker panel consisting of anti-MMP3, anti-PRDX2, anti-SPARC, and anti-HSPA5 for detection of early-stage OSCC achieved a sensitivity of 63.8% with a specificity of 90%. Collectively, herein we established a multiplex auto-Ab platform for OSCC screening, and demonstrated a four-auto-Ab panel which shows clinical applicability for early diagnosis of OSCC.
Keywords: autoantibody; biomarker; cancer screening; oral cancer; saliva.
Copyright © 2022 Hsueh, Chang, Liu, Chiang, Chan, Hung, Chu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Salivary auto-antibodies as noninvasive diagnostic markers of oral cavity squamous cell carcinoma.Cancer Epidemiol Biomarkers Prev. 2014 Aug;23(8):1569-78. doi: 10.1158/1055-9965.EPI-13-1269. Epub 2014 May 23. Cancer Epidemiol Biomarkers Prev. 2014. PMID: 24859869
-
Identification of salivary autoantibodies as biomarkers of oral cancer with immunoglobulin A enrichment combined with affinity mass spectrometry.Proteomics. 2023 May;23(9):e2200321. doi: 10.1002/pmic.202200321. Epub 2023 Jan 18. Proteomics. 2023. PMID: 36625099
-
An immuno-MALDI mass spectrometry assay for the oral cancer biomarker, matrix metalloproteinase-1, in dried saliva spot samples.Anal Chim Acta. 2020 Mar 1;1100:118-130. doi: 10.1016/j.aca.2019.12.006. Epub 2019 Dec 3. Anal Chim Acta. 2020. PMID: 31987131
-
Salivary Biomarkers in Oral Squamous Cell Carcinoma: A Proteomic Overview.Proteomes. 2022 Nov 7;10(4):37. doi: 10.3390/proteomes10040037. Proteomes. 2022. PMID: 36412636 Free PMC article. Review.
-
Could salivary biomarkers be useful in the early detection of oral cancer and oral potentially malignant disorders, and is there a relationship between these biomarkers and risk factors?Evid Based Dent. 2022 Mar;23(1):30-31. doi: 10.1038/s41432-022-0249-8. Epub 2022 Mar 25. Evid Based Dent. 2022. PMID: 35338326 Review.
Cited by
-
Biological biomarkers of oral cancer.Periodontol 2000. 2023 Dec 10:10.1111/prd.12542. doi: 10.1111/prd.12542. Online ahead of print. Periodontol 2000. 2023. PMID: 38073011 Review.
-
Identification of novel serum autoantibody biomarkers for early esophageal squamous cell carcinoma and high-grade intraepithelial neoplasia detection.Front Oncol. 2023 May 12;13:1161489. doi: 10.3389/fonc.2023.1161489. eCollection 2023. Front Oncol. 2023. PMID: 37251926 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous


