Novel Natural Compounds and Their Anatomical Distribution in the Stinging Fireworm Hermodice carunculata (Annelida)
- PMID: 36135774
- PMCID: PMC9504318
- DOI: 10.3390/md20090585
Novel Natural Compounds and Their Anatomical Distribution in the Stinging Fireworm Hermodice carunculata (Annelida)
Abstract
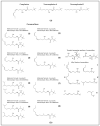
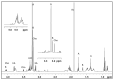
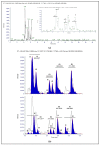
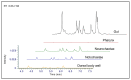
Increasing evidence in the field of bioprospection fosters the necessity of studying poorly investigated poisonous marine invertebrates to expand knowledge on animal venom biology. Among marine annelids, amphinomid fireworms are notorious for their bearded trunk equipped with a powerful stinging capacity. Here, a methodological workflow based on analytical chemistry techniques (compound isolation followed by mass spectrometry and spectroscopy analyses) was applied to gain new insights, leading to the identification and structural elucidation of an array of natural products from Mediterranean specimens of Hermodice carunculata. Eight betaine-derived unprecedented compounds, named "carunculines", were detected, bearing two terminal ammonium groups tri-and disubstituted at the Cα (A, B) and a series of different alkyl chains (I-VIII). The mixture of chemicals was found in all the body parts of H. carunculata, supporting a mechanism of action triggered by their vehiculation inside the dorsal chaetae, and subsequent injection when chaetae break off on contact. Preliminary investigations to understand adaptive features were also performed, showing a trend in carunculine abundance that fits into the evolutionary history of these worms. These findings shed light on the chemical ecology of amphinomids, giving reasons for the success of H. carunculata in benthic environments and providing promising novel metabolites for biotechnological implications.
Keywords: amphinomid; aposematism; chemical defences; marine natural products; phylogeny.
Conflict of interest statement
The authors declare that they have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Commentary on: "Unravelling the ultrastructure and mineralogical composition of fireworm stinging bristles" by Righi et al. 2020.Zoology (Jena). 2021 Feb;144:125890. doi: 10.1016/j.zool.2020.125890. Epub 2020 Dec 16. Zoology (Jena). 2021. PMID: 33451887
-
Response to Tilic and Bartolomaeus's Commentary on the original Research Paper "Unravelling the ultrastructure and mineralogical composition of fireworm stinging bristles" (Zoology, 144).Zoology (Jena). 2021 Feb;144:125889. doi: 10.1016/j.zool.2020.125889. Epub 2020 Dec 17. Zoology (Jena). 2021. PMID: 33454148
-
Regeneration of posterior segments and terminal structures in the bearded fireworm, Hermodice carunculata (Annelida: Amphinomidae).J Morphol. 2014 Oct;275(10):1103-12. doi: 10.1002/jmor.20287. Epub 2014 May 5. J Morphol. 2014. PMID: 24796944
-
The hidden biotechnological potential of marine invertebrates: The Polychaeta case study.Environ Res. 2019 Jun;173:270-280. doi: 10.1016/j.envres.2019.03.048. Epub 2019 Mar 22. Environ Res. 2019. PMID: 30928858 Review.
-
Is Diurodrilus an annelid?J Morphol. 2008 Dec;269(12):1426-55. doi: 10.1002/jmor.10686. J Morphol. 2008. PMID: 18985766 Review.
Cited by
-
Highly Concentrated Linear Guanidine Amides from the Marine Sipunculid Phascolosoma granulatum.J Nat Prod. 2024 Apr 26;87(4):906-913. doi: 10.1021/acs.jnatprod.3c01186. Epub 2024 Mar 2. J Nat Prod. 2024. PMID: 38430199 Free PMC article.
References
-
- Pawlik J.R. Antipredatory defensive roles of natural products from marine invertebrates. In: Fattorusso E., Gerwick W.H., Taglialatela-Scafati O., editors. Handbook of Marine Natural Products. Springer; Dordrecht, the Netherlands: 2012. pp. 677–710. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


