Regulation of signaling pathways in hair follicle stem cells
- PMID: 35795256
- PMCID: PMC9250793
- DOI: 10.1093/burnst/tkac022
Regulation of signaling pathways in hair follicle stem cells
Abstract
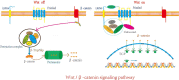
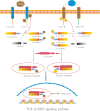
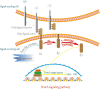
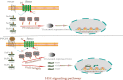
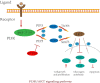
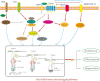
Hair follicle stem cells (HFSCs) reside in the bulge region of the outer root sheath of the hair follicle. They are considered slow-cycling cells that are endowed with multilineage differentiation potential and superior proliferative capacity. The normal morphology and periodic growth of HFSCs play a significant role in normal skin functions, wound repair and skin regeneration. The HFSCs involved in these pathophysiological processes are regulated by a series of cell signal transduction pathways, such as lymphoid enhancer factor/T-cell factor, Wnt/β-catenin, transforming growth factor-β/bone morphogenetic protein, Notch and Hedgehog. The mechanisms of the interactions among these signaling pathways and their regulatory effects on HFSCs have been previously studied, but many mechanisms are still unclear. This article reviews the regulation of hair follicles, HFSCs and related signaling pathways, with the aims of summarizing previous research results, revealing the regulatory mechanisms of HFSC proliferation and differentiation and providing important references and new ideas for treating clinical diseases.
Keywords: Differentiation; Hair follicle stem cells; Proliferation; Regenerative; Repair; Signaling pathways.
© The Author(s) 2022. Published by Oxford University Press.
Figures
Similar articles
-
A systematic summary of survival and death signalling during the life of hair follicle stem cells.Stem Cell Res Ther. 2021 Aug 11;12(1):453. doi: 10.1186/s13287-021-02527-y. Stem Cell Res Ther. 2021. PMID: 34380571 Free PMC article. Review.
-
Nanoscale microenvironment engineering for expanding human hair follicle stem cell and revealing their plasticity.J Nanobiotechnology. 2021 Mar 31;19(1):94. doi: 10.1186/s12951-021-00840-5. J Nanobiotechnology. 2021. PMID: 33789665 Free PMC article.
-
Low-level laser activates Wnt/β-catenin signaling pathway-promoting hair follicle stem cell regeneration and wound healing: Upregulate the expression of key downstream gene Lef 1.Skin Res Technol. 2024 Jun;30(6):e13807. doi: 10.1111/srt.13807. Skin Res Technol. 2024. PMID: 38887112 Free PMC article.
-
BMP-AKT-GSK3β Signaling Restores Hair Follicle Stem Cells Decrease Associated with Loss of Sfrp1.Stem Cells. 2022 Sep 26;40(9):802-817. doi: 10.1093/stmcls/sxac041. Stem Cells. 2022. PMID: 35689817
-
Beauty is skin deep: the fascinating biology of the epidermis and its appendages.Harvey Lect. 1998-1999;94:47-77. Harvey Lect. 1998. PMID: 11070952 Review.
Cited by
-
Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. cv. Buebang 3 CMU and Sanpatong.Plants (Basel). 2024 Nov 1;13(21):3079. doi: 10.3390/plants13213079. Plants (Basel). 2024. PMID: 39519997 Free PMC article.
-
Exosomes derived from Umbilical cord mesenchymal stem cell promote hair regrowth in C57BL6 mice through upregulation of the RAS/ERK signaling pathway.J Transl Int Med. 2024 Nov 6;12(5):478-494. doi: 10.1515/jtim-2024-0012. eCollection 2024 Nov. J Transl Int Med. 2024. PMID: 39513036 Free PMC article.
-
Molecular Interplay in Cardiac Fibrosis: Exploring the Functions of RUNX2, BMP2, and Notch.Rev Cardiovasc Med. 2024 Oct 16;25(10):368. doi: 10.31083/j.rcm2510368. eCollection 2024 Oct. Rev Cardiovasc Med. 2024. PMID: 39484128 Free PMC article. Review.
-
CRABP1 Enhances the Proliferation of the Dermal Papilla Cells of Hu Sheep through the Wnt/β-catenin Pathway.Genes (Basel). 2024 Sep 30;15(10):1291. doi: 10.3390/genes15101291. Genes (Basel). 2024. PMID: 39457415 Free PMC article.
-
Synergistic Phytochemical and Pharmacological Actions of Hair RiseTM Microemulsion: A Novel Herbal Formulation for Androgenetic Alopecia and Hair Growth Stimulation.Plants (Basel). 2024 Oct 6;13(19):2802. doi: 10.3390/plants13192802. Plants (Basel). 2024. PMID: 39409672 Free PMC article.
References
-
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–7. - PubMed
-
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. - PubMed
-
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. - PubMed
-
- Nicu C, Wikramanayake TC, Paus R. Clues that mitochondria are involved in the hair cycle clock: MPZL3 regulates entry into and progression of murine hair follicle cycling. Exp Dermatol. 2020;29:1243–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources


