Testicular ultrastructure and hormonal changes following administration of tenofovir disoproxil fumarate-loaded silver nanoparticle in type-2 diabetic rats
- PMID: 35688844
- PMCID: PMC9187647
- DOI: 10.1038/s41598-022-13321-y
Testicular ultrastructure and hormonal changes following administration of tenofovir disoproxil fumarate-loaded silver nanoparticle in type-2 diabetic rats
Abstract
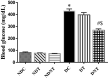
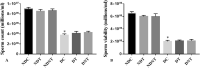
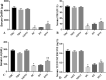
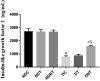
Reproductive dysfunctions (RDs) characterized by impairment in testicular parameters, and metabolic disorders such as insulin resistance and type 2 diabetes mellitus (T2DM) are on the rise among human immunodeficiency virus (HIV) patients under tenofovir disoproxil fumarate (TDF) and highly active antiretroviral therapy (HAART). These adverse effects require a nanoparticle delivery system to circumvent biological barriers and ensure adequate ARVDs to viral reservoir sites like testis. This study aimed to investigate the effect of TDF-loaded silver nanoparticles (AgNPs), TDF-AgNPs on sperm quality, hormonal profile, insulin-like growth factor 1 (IGF-1), and testicular ultrastructure in diabetic rats, a result of which could cater for the neglected reproductive and metabolic dysfunctions in HIV therapeutic modality. Thirty-six adult Sprague-Dawley rats were assigned to diabetic and non-diabetic (n = 18). T2DM was induced by fructose-streptozotocin (Frt-STZ) rat model. Subsequently, the rats in both groups were subdivided into three groups each (n = 6) and administered distilled water, TDF, and TDF-AgNP. In this study, administration of TDF-AgNP to diabetic rats significantly reduced (p < 0.05) blood glucose level (268.7 ± 10.8 mg/dL) from 429 ± 16.9 mg/dL in diabetic control and prevented a drastic reduction in sperm count and viability. More so, TDF-AgNP significantly increased (p < 0.05) Gonadotropin-Releasing Hormone (1114.3 ± 112.6 µg), Follicle Stimulating Hormone (13.2 ± 1.5 IU/L), Luteinizing Hormone (140.7 ± 15.2 IU/L), testosterone (0.2 ± 0.02 ng/L), and IGF-1 (1564.0 ± 81.6 ng/mL) compared to their respective diabetic controls (383.4 ± 63.3, 6.1 ± 1.2, 76.1 ± 9.1, 0.1 ± 0.01, 769.4 ± 83.7). Also, TDF-AgNP treated diabetic rats presented an improved testicular architecture marked with the thickened basement membrane, degenerated Sertoli cells, spermatogenic cells, and axoneme. This study has demonstrated that administration of TDF-AgNPs restored the function of hypothalamic-pituitary-gonadal axis, normalized the hormonal profile, enhanced testicular function and structure to alleviate reproductive dysfunctions in diabetic rats. This is the first study to conjugate TDF with AgNPs and examined its effects on reproductive indices, local gonadal factor and testicular ultrastructure in male diabetic rats with the potential to cater for neglected reproductive dysfunction in HIV therapeutic modality.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Evaluation of tenofovir disoproxil fumarate loaded silver nanoparticle on testicular morphology in experimental type-2 diabetic rats.Artif Cells Nanomed Biotechnol. 2022 Dec;50(1):71-80. doi: 10.1080/21691401.2022.2042009. Artif Cells Nanomed Biotechnol. 2022. PMID: 35343349
-
Studies on testicular ultrastructural and hormonal changes in type-2 diabetic rats treated with highly active antiretroviral therapy conjugated silver nanoparticles.Life Sci. 2022 Jun 1;298:120498. doi: 10.1016/j.lfs.2022.120498. Epub 2022 Mar 24. Life Sci. 2022. PMID: 35341824
-
Highly active antiretroviral therapy conjugated silver nanoparticle ameliorates testicular injury in type-2 diabetic rats.Heliyon. 2021 Dec 9;7(12):e08580. doi: 10.1016/j.heliyon.2021.e08580. eCollection 2021 Dec. Heliyon. 2021. PMID: 34917828 Free PMC article.
-
Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: A meta-analysis of randomized controlled trials.Int J Infect Dis. 2020 Apr;93:108-117. doi: 10.1016/j.ijid.2020.01.035. Epub 2020 Jan 25. Int J Infect Dis. 2020. PMID: 31988012 Review.
-
Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).Biochem Pharmacol. 2016 Nov 1;119:1-7. doi: 10.1016/j.bcp.2016.04.015. Epub 2016 Apr 29. Biochem Pharmacol. 2016. PMID: 27133890 Review.
Cited by
-
Histomorphometric changes in testis following administration of tenofovir nanoparticles in an animal model.Discov Nano. 2024 Mar 25;19(1):56. doi: 10.1186/s11671-024-04002-y. Discov Nano. 2024. PMID: 38526666 Free PMC article.
-
Targeting metabolic pathways: a novel therapeutic direction for type 2 diabetes.Front Cell Infect Microbiol. 2023 Aug 2;13:1218326. doi: 10.3389/fcimb.2023.1218326. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37600949 Free PMC article.
-
Nanodelivery of antiretroviral drugs to nervous tissues.Front Pharmacol. 2022 Nov 8;13:1025160. doi: 10.3389/fphar.2022.1025160. eCollection 2022. Front Pharmacol. 2022. PMID: 36425574 Free PMC article. Review.
References
-
- Collazos J. Sexual dysfunction in the highly active antiretroviral therapy era. AIDS Rev. 2007;9(4):237–245. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous


