Altered neural cell junctions and ion-channels leading to disrupted neuron communication in Parkinson's disease
- PMID: 35650269
- PMCID: PMC9160246
- DOI: 10.1038/s41531-022-00324-9
Altered neural cell junctions and ion-channels leading to disrupted neuron communication in Parkinson's disease
Abstract
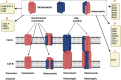
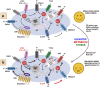
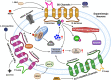
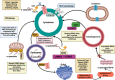
Parkinson's disease (PD) is a neurological disorder that affects the movement of the human body. It is primarily characterized by reduced dopamine levels in the brain. The causative agent of PD is still unclear but it is generally accepted that α-synuclein has a central role to play. It is also known that gap-junctions and associated connexins are complicated structures that play critical roles in nervous system signaling and associated misfunctioning. Thus, our current article emphasizes how, alongside α-synuclein, ion-channels, gap-junctions, and related connexins, all play vital roles in influencing multiple metabolic activities of the brain during PD. It also highlights that ion-channel and gap-junction disruptions, which are primarily mediated by their structural-functional changes and alterations, have a role in PD. Furthermore, we discussed available drugs and advanced therapeutic interventions that target Parkinson's pathogenesis. In conclusion, it warrants creating better treatments for PD patients. Although, dopaminergic replenishment therapy is useful in treating neurological problems, such therapies are, however, unable to control the degeneration that underpins the disease, thereby declining their overall efficacy. This creates an additional challenge and an untapped scope for neurologists to adopt treatments for PD by targeting the ion-channels and gap-junctions, which is well-reviewed in the present article.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Potassium Channels in Parkinson's Disease: Potential Roles in Its Pathogenesis and Innovative Molecular Targets for Treatment.Pharmacol Rev. 2023 Jul;75(4):758-788. doi: 10.1124/pharmrev.122.000743. Epub 2023 Mar 14. Pharmacol Rev. 2023. PMID: 36918260 Review.
-
The role and therapeutic potential of connexins, pannexins and their channels in Parkinson's disease.Cell Signal. 2019 Jun;58:111-118. doi: 10.1016/j.cellsig.2019.03.010. Epub 2019 Mar 12. Cell Signal. 2019. PMID: 30877035 Review.
-
Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson's Disease.J Neurosci. 2016 Apr 6;36(14):4026-37. doi: 10.1523/JNEUROSCI.1395-15.2016. J Neurosci. 2016. PMID: 27053209 Free PMC article.
-
Reduced dopaminergic neuron degeneration and global transcriptional changes in Parkinson's disease mouse brains engrafted with human neural stems during the early disease stage.Exp Neurol. 2022 Jun;352:114042. doi: 10.1016/j.expneurol.2022.114042. Epub 2022 Mar 8. Exp Neurol. 2022. PMID: 35271839
-
Translational molecular imaging and drug development in Parkinson's disease.Mol Neurodegener. 2023 Feb 10;18(1):11. doi: 10.1186/s13024-023-00600-z. Mol Neurodegener. 2023. PMID: 36759912 Free PMC article. Review.
Cited by
-
Brain-Region-Specific Differences in Protein Citrullination/Deimination in a Pre-Motor Parkinson's Disease Rat Model.Int J Mol Sci. 2024 Oct 17;25(20):11168. doi: 10.3390/ijms252011168. Int J Mol Sci. 2024. PMID: 39456949 Free PMC article.
-
Gut microbiota defined epigenomes of Alzheimer's and Parkinson's diseases reveal novel targets for therapy.Epigenomics. 2024 Jan;16(1):57-77. doi: 10.2217/epi-2023-0342. Epub 2023 Dec 13. Epigenomics. 2024. PMID: 38088063 Review.
-
Restoring autophagic function: a case for type 2 diabetes mellitus drug repurposing in Parkinson's disease.Front Neurosci. 2023 Nov 3;17:1244022. doi: 10.3389/fnins.2023.1244022. eCollection 2023. Front Neurosci. 2023. PMID: 38027497 Free PMC article. Review.
-
Alpha-synuclein null mutation exacerbates the phenotype of a model of Menkes disease in female mice.bioRxiv [Preprint]. 2023 Nov 17:2023.11.15.567255. doi: 10.1101/2023.11.15.567255. bioRxiv. 2023. PMID: 38014334 Free PMC article. Preprint.
-
Whole Transcriptome Analysis of Substantia Nigra in Mice with MPTP-Induced Parkinsonism Bearing Defective Glucocerebrosidase Activity.Int J Mol Sci. 2023 Jul 29;24(15):12164. doi: 10.3390/ijms241512164. Int J Mol Sci. 2023. PMID: 37569538 Free PMC article.
References
-
- Kalinderi, K., Bostantjopoulou, S. & Fidani, L. The genetic background of Parkinson’s disease: current progress and future prospects. Acta. Neurol. Scand.134, 314–326 (2016). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous


