Intercropping Walnut and Tea: Effects on Soil Nutrients, Enzyme Activity, and Microbial Communities
- PMID: 35369467
- PMCID: PMC8971985
- DOI: 10.3389/fmicb.2022.852342
Intercropping Walnut and Tea: Effects on Soil Nutrients, Enzyme Activity, and Microbial Communities
Abstract
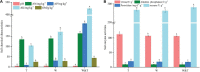
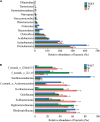
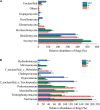
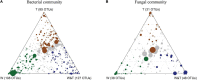
The practice of intercropping, which involves growing more than one crop simultaneously during the same growing season, is becoming more important for increasing soil quality, land-use efficiency, and subsequently crop productivity. The present study examined changes in soil physicochemical properties, enzymatic activity, and microbial community composition when walnut (Juglans spp.) was intercropped with tea (Camellia sinensis L.) plants in a forest and compared with a walnut and tea monocropping system. The results showed that walnut-tea intercropping improved the soil nutrient profile and enzymatic activity. The soil available nitrogen (AN), available phosphorus (AP), available potassium (AK), organic matter (OM) content, and sucrase activity were significantly boosted in intercropped walnut and tea than in monocropping forests. The interaction between crops further increased bacterial and fungal diversity when compared to monoculture tea forests. Proteobacteria, Bacteroidetes, Firmicutes, Chlamydiae, Rozellomycota, and Zoopagomycota were found in greater abundance in an intercropping pattern than in monoculture walnut and tea forest plantations. The walnut-tea intercropping system also markedly impacted the abundance of several bacterial and fungal operational taxonomic units (OTUs), which were previously shown to support nutrient cycling, prevent diseases, and ameliorate abiotic stress. The results of this study suggest that intercropping walnut with tea increased host fitness and growth by positively influencing soil microbial populations.
Keywords: bacterial community; beneficial microbiota; fungal community; intercropping; microbial diversity; soil nutrients.
Copyright © 2022 Bai, Li, Xu, Raza, Wang, Wang, Fu, Hu, Imoulan, Hussain and Xu.
Conflict of interest statement
J-YH is employed by Shenyang Sinochem Agrochemicals R&D Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Impact of Intercropping Five Medicinal Plants on Soil Nutrients, Enzyme Activity, and Microbial Community Structure in Camellia oleifera Plantations.Microorganisms. 2024 Aug 8;12(8):1616. doi: 10.3390/microorganisms12081616. Microorganisms. 2024. PMID: 39203458 Free PMC article.
-
Intercropping system modulated soil-microbe interactions that enhanced the growth and quality of flue-cured tobacco by improving rhizospheric soil nutrients, microbial structure, and enzymatic activities.Front Plant Sci. 2023 Oct 24;14:1233464. doi: 10.3389/fpls.2023.1233464. eCollection 2023. Front Plant Sci. 2023. PMID: 37941660 Free PMC article.
-
Dynamic changes of rhizosphere soil bacterial community and nutrients in cadmium polluted soils with soybean-corn intercropping.BMC Microbiol. 2022 Feb 15;22(1):57. doi: 10.1186/s12866-022-02468-3. BMC Microbiol. 2022. PMID: 35168566 Free PMC article.
-
Intercropping of Peanut-Tea Enhances Soil Enzymatic Activity and Soil Nutrient Status at Different Soil Profiles in Subtropical Southern China.Plants (Basel). 2021 Apr 27;10(5):881. doi: 10.3390/plants10050881. Plants (Basel). 2021. PMID: 33925476 Free PMC article.
-
Intercropping Cover Crops for a Vital Ecosystem Service: A Review of the Biocontrol of Insect Pests in Tea Agroecosystems.Plants (Basel). 2023 Jun 18;12(12):2361. doi: 10.3390/plants12122361. Plants (Basel). 2023. PMID: 37375986 Free PMC article. Review.
Cited by
-
A Novel Plant-Derived Biopesticide Mitigates Fusarium Root Rot of Angelica sinensis by Modulating the Rhizosphere Microbiome and Root Metabolome.Plants (Basel). 2024 Aug 6;13(16):2180. doi: 10.3390/plants13162180. Plants (Basel). 2024. PMID: 39204616 Free PMC article.
-
Impact of Intercropping Five Medicinal Plants on Soil Nutrients, Enzyme Activity, and Microbial Community Structure in Camellia oleifera Plantations.Microorganisms. 2024 Aug 8;12(8):1616. doi: 10.3390/microorganisms12081616. Microorganisms. 2024. PMID: 39203458 Free PMC article.
-
Hexose/pentose ratio in rhizosphere exudates-mediated soil eutrophic/oligotrophic bacteria regulates the growth pattern of host plant in young apple-aromatic plant intercropping systems.Front Microbiol. 2024 Mar 25;15:1364355. doi: 10.3389/fmicb.2024.1364355. eCollection 2024. Front Microbiol. 2024. PMID: 38591033 Free PMC article.
-
Intercropping system modulated soil-microbe interactions that enhanced the growth and quality of flue-cured tobacco by improving rhizospheric soil nutrients, microbial structure, and enzymatic activities.Front Plant Sci. 2023 Oct 24;14:1233464. doi: 10.3389/fpls.2023.1233464. eCollection 2023. Front Plant Sci. 2023. PMID: 37941660 Free PMC article.
-
Legume-grass mixtures increase forage yield by improving soil quality in different ecological regions of the Qinghai-Tibet Plateau.Front Plant Sci. 2023 Oct 19;14:1280771. doi: 10.3389/fpls.2023.1280771. eCollection 2023. Front Plant Sci. 2023. PMID: 37929174 Free PMC article.
References
-
- Abbasi S. A., Nazari M., Fallah S., Iranipour R., Mousavi A. (2020). The competitive effect of almond trees on light and nutrients absorption, crop growth rate, and the yield in almond–cereal agroforestry systems in semi-arid regions. Agroforest. Syst. 94 1111–1122. 10.1007/s10457-019-00469-2 - DOI
-
- Ahmed A., Aftab S., Hussain S., Nazir Cheema H., Liu W. G., Yang F., et al. (2020). Nutrient accumulation and distribution assessment in response to potassium application under maize–soybean intercropping system. Agronomy 10:725. 10.3390/agronomy10050725 - DOI
-
- Arenas-Corraliza M. G., López-Díaz M. L., Moreno G. (2018). Winter cereal production in a Mediterranean silvoarable walnut system in the face of climate change. Agr. Ecosyst. Environ. 264 111–118. 10.1016/j.agee.2018.05.024 - DOI
-
- Bach C. E., Warnock D. D., Van Horn D. J., Weintraub M. N., Sinsabaugh R. L., Allison S. D., et al. (2013). Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: effect of assay conditions and soil type. Soil Biol. Biochem. 67 183–191. 10.1016/j.soilbio.2013.08.022 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous


