Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease
- PMID: 35162986
- PMCID: PMC8834714
- DOI: 10.3390/ijms23031062
Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease
Abstract
Metabolic-associated fatty liver disease (MAFLD), which is often linked to obesity, encompasses a large spectrum of hepatic lesions, including simple fatty liver, steatohepatitis, cirrhosis and hepatocellular carcinoma. Besides nutritional and genetic factors, different xenobiotics such as pharmaceuticals and environmental toxicants are suspected to aggravate MAFLD in obese individuals. More specifically, pre-existing fatty liver or steatohepatitis may worsen, or fatty liver may progress faster to steatohepatitis in treated patients, or exposed individuals. The mechanisms whereby xenobiotics can aggravate MAFLD are still poorly understood and are currently under deep investigations. Nevertheless, previous studies pointed to the role of different metabolic pathways and cellular events such as activation of de novo lipogenesis and mitochondrial dysfunction, mostly associated with reactive oxygen species overproduction. This review presents the available data gathered with some prototypic compounds with a focus on corticosteroids and rosiglitazone for pharmaceuticals as well as bisphenol A and perfluorooctanoic acid for endocrine disruptors. Although not typically considered as a xenobiotic, ethanol is also discussed because its abuse has dire consequences on obese liver.
Keywords: NASH; drugs; endocrine disruptors; environmental contaminants; ethanol; fatty liver; obesity.
Conflict of interest statement
J. Massart, K. Begriche, A. Corlu and B. Fromenty declare that they have no conflict of interest in relation to this work.
Figures
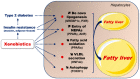
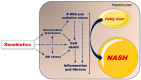
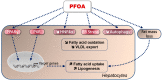
 : increase;
: increase;  : decrease).
: decrease).Similar articles
-
Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury?Biochimie. 2020 Dec;179:266-274. doi: 10.1016/j.biochi.2020.08.018. Epub 2020 Sep 3. Biochimie. 2020. PMID: 32891697 Free PMC article. Review.
-
Drug-induced liver injury in obesity and nonalcoholic fatty liver disease.Adv Pharmacol. 2019;85:75-107. doi: 10.1016/bs.apha.2019.01.003. Epub 2019 Feb 20. Adv Pharmacol. 2019. PMID: 31307592 Review.
-
Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways.Clin Sci (Lond). 2022 Sep 30;136(18):1347-1366. doi: 10.1042/CS20220572. Clin Sci (Lond). 2022. PMID: 36148775 Free PMC article. Review.
-
Infections at the nexus of metabolic-associated fatty liver disease.Arch Toxicol. 2021 Jul;95(7):2235-2253. doi: 10.1007/s00204-021-03069-1. Epub 2021 May 24. Arch Toxicol. 2021. PMID: 34027561 Free PMC article. Review.
-
Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations.World J Gastroenterol. 2014 Jul 28;20(28):9330-7. doi: 10.3748/wjg.v20.i28.9330. World J Gastroenterol. 2014. PMID: 25071327 Free PMC article. Review.
Cited by
-
Liver and Pancreatic Toxicity of Endocrine-Disruptive Chemicals: Focus on Mitochondrial Dysfunction and Oxidative Stress.Int J Mol Sci. 2024 Jul 6;25(13):7420. doi: 10.3390/ijms25137420. Int J Mol Sci. 2024. PMID: 39000526 Free PMC article. Review.
-
The Interplay between Endocrine-Disrupting Chemicals and the Epigenome towards Metabolic Dysfunction-Associated Steatotic Liver Disease: A Comprehensive Review.Nutrients. 2024 Apr 11;16(8):1124. doi: 10.3390/nu16081124. Nutrients. 2024. PMID: 38674815 Free PMC article. Review.
-
Real-time fluorescent monitoring of phase I xenobiotic-metabolizing enzymes.RSC Adv. 2024 Mar 15;14(13):8837-8870. doi: 10.1039/d4ra00127c. eCollection 2024 Mar 14. RSC Adv. 2024. PMID: 38495994 Free PMC article. Review.
-
MKP1 promotes nonalcoholic steatohepatitis by suppressing AMPK activity through LKB1 nuclear retention.Nat Commun. 2023 Sep 5;14(1):5405. doi: 10.1038/s41467-023-41145-5. Nat Commun. 2023. PMID: 37669951 Free PMC article.
-
Thoughts on Non-Alcoholic Fatty Liver Disease and Chronic Disease.Integr Med (Encinitas). 2023 May;22(2):6-9. Integr Med (Encinitas). 2023. PMID: 37363148 Free PMC article.
References
-
- Eslam M., Sanyal A.J., George J., Sanyal A., Neuschwander-Tetri B., Tiribelli C., Kleiner D.E., Brunt E., Bugianesi E., Yki-Järvinen H., et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


