Rapid SARS-CoV-2 Adaptation to Available Cellular Proteases
- PMID: 35019723
- PMCID: PMC8906416
- DOI: 10.1128/jvi.02186-21
Rapid SARS-CoV-2 Adaptation to Available Cellular Proteases
Abstract
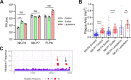
Recent emergence of SARS-CoV-1 variants demonstrates the potential of this virus for targeted evolution, despite its overall genomic stability. Here we show the dynamics and the mechanisms behind the rapid adaptation of SARS-CoV-2 to growth in Vero E6 cells. The selective advantage for growth in Vero E6 cells is due to increased cleavage efficiency by cathepsins at the mutated S1/S2 site. S1/S2 site also constitutes a heparan sulfate (HS) binding motif that influenced virus growth in Vero E6 cells, but HS antagonist did not inhibit virus adaptation in these cells. The entry of Vero E6-adapted virus into human cells is defective because the mutated spike variants are poorly processed by furin or TMPRSS2. Minor subpopulation that lack the furin cleavage motif in the spike protein rapidly become dominant upon passaging through Vero E6 cells, but wild type sequences are maintained at low percentage in the virus swarm and mediate a rapid reverse adaptation if the virus is passaged again on TMPRSS2+ human cells. Our data show that the spike protein of SARS-CoV-2 can rapidly adapt itself to available proteases and argue for deep sequence surveillance to identify the emergence of novel variants. IMPORTANCE Recently emerging SARS-CoV-2 variants B.1.1.7 (alpha variant), B.1.617.2 (delta variant), and B.1.1.529 (omicron variant) harbor spike mutations and have been linked to increased virus pathogenesis. The emergence of these novel variants highlights coronavirus adaptation and evolution potential, despite the stable consensus genotype of clinical isolates. We show that subdominant variants maintained in the virus population enable the virus to rapidly adapt to selection pressure. Although these adaptations lead to genotype change, the change is not absolute and genomes with original genotype are maintained in the virus swarm. Thus, our results imply that the relative stability of SARS-CoV-2 in numerous independent clinical isolates belies its potential for rapid adaptation to new conditions.
Keywords: SARS-CoV-2; coronavirus spike priming; deep sequencing; furin cleavage site; spike mutation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets.Nat Microbiol. 2021 Jul;6(7):899-909. doi: 10.1038/s41564-021-00908-w. Epub 2021 Apr 27. Nat Microbiol. 2021. PMID: 33907312
-
Natural and Recombinant SARS-CoV-2 Isolates Rapidly Evolve In Vitro to Higher Infectivity through More Efficient Binding to Heparan Sulfate and Reduced S1/S2 Cleavage.J Virol. 2021 Oct 13;95(21):e0135721. doi: 10.1128/JVI.01357-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406867 Free PMC article.
-
Vero cell-adapted SARS-CoV-2 strain shows increased viral growth through furin-mediated efficient spike cleavage.Microbiol Spectr. 2024 Apr 2;12(4):e0285923. doi: 10.1128/spectrum.02859-23. Epub 2024 Feb 28. Microbiol Spectr. 2024. PMID: 38415690 Free PMC article.
-
Proteolytic activation of SARS-CoV-2 spike protein.Microbiol Immunol. 2022 Jan;66(1):15-23. doi: 10.1111/1348-0421.12945. Epub 2021 Oct 12. Microbiol Immunol. 2022. PMID: 34561887 Free PMC article. Review.
-
Contributions of human ACE2 and TMPRSS2 in determining host-pathogen interaction of COVID-19.J Genet. 2021;100(1):12. doi: 10.1007/s12041-021-01262-w. J Genet. 2021. PMID: 33707363 Free PMC article. Review.
Cited by
-
A single-dose MCMV-based vaccine elicits long-lasting immune protection in mice against distinct SARS-CoV-2 variants.Front Immunol. 2024 Jul 25;15:1383086. doi: 10.3389/fimmu.2024.1383086. eCollection 2024. Front Immunol. 2024. PMID: 39119342 Free PMC article.
-
An inactivated SARS-CoV-2 vaccine based on a Vero cell culture-adapted high-titer virus confers cross-protection in small animals.Sci Rep. 2024 Jul 24;14(1):17039. doi: 10.1038/s41598-024-67570-0. Sci Rep. 2024. PMID: 39048693 Free PMC article.
-
Cell type-specific adaptation of the SARS-CoV-2 spike.Virus Evol. 2024 Apr 25;10(1):veae032. doi: 10.1093/ve/veae032. eCollection 2024. Virus Evol. 2024. PMID: 38779130 Free PMC article.
-
Temperature impacts SARS-CoV-2 spike fusogenicity and evolution.mBio. 2024 Apr 10;15(4):e0336023. doi: 10.1128/mbio.03360-23. Epub 2024 Feb 27. mBio. 2024. PMID: 38411986 Free PMC article.
-
Using big sequencing data to identify chronic SARS-Coronavirus-2 infections.Nat Commun. 2024 Jan 20;15(1):648. doi: 10.1038/s41467-024-44803-4. Nat Commun. 2024. PMID: 38245511 Free PMC article.
References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus I, Research T . 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. 2020. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280. 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


