How the Chemical Properties of GBCAs Influence Their Safety Profiles In Vivo
- PMID: 35011290
- PMCID: PMC8746842
- DOI: 10.3390/molecules27010058
How the Chemical Properties of GBCAs Influence Their Safety Profiles In Vivo
Abstract
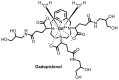
The extracellular class of gadolinium-based contrast agents (GBCAs) is an essential tool for clinical diagnosis and disease management. In order to better understand the issues associated with GBCA administration and gadolinium retention and deposition in the human brain, the chemical properties of GBCAs such as relative thermodynamic and kinetic stabilities and their likelihood of forming gadolinium deposits in vivo will be reviewed. The chemical form of gadolinium causing the hyperintensity is an open question. On the basis of estimates of total gadolinium concentration present, it is highly unlikely that the intact chelate is causing the T1 hyperintensities observed in the human brain. Although it is possible that there is a water-soluble form of gadolinium that has high relaxitvity present, our experience indicates that the insoluble gadolinium-based agents/salts could have high relaxivities on the surface of the solid due to higher water access. This review assesses the safety of GBCAs from a chemical point of view based on their thermodynamic and kinetic properties, discusses how these properties influence in vivo behavior, and highlights some clinical implications regarding the development of future imaging agents.
Keywords: T1 hyperintensity; gadolinium deposition; gadolinium-based contrast agents; kinetic inertness; thermodynamic stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
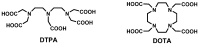

Similar articles
-
Quantification and Assessment of the Chemical Form of Residual Gadolinium in the Brain After Repeated Administration of Gadolinium-Based Contrast Agents: Comparative Study in Rats.Invest Radiol. 2017 Jul;52(7):396-404. doi: 10.1097/RLI.0000000000000352. Invest Radiol. 2017. PMID: 28125438 Free PMC article.
-
Gadolinium Deposition in the Brain: Current Updates.Korean J Radiol. 2019 Jan;20(1):134-147. doi: 10.3348/kjr.2018.0356. Epub 2018 Dec 27. Korean J Radiol. 2019. PMID: 30627029 Free PMC article. Review.
-
Thermodynamic stability and kinetic inertness of a Gd-DTPA bisamide complex grafted onto gold nanoparticles.Contrast Media Mol Imaging. 2015 May-Jun;10(3):179-87. doi: 10.1002/cmmi.1616. Epub 2014 Aug 8. Contrast Media Mol Imaging. 2015. PMID: 25130910
-
Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review.Biometals. 2008 Aug;21(4):469-90. doi: 10.1007/s10534-008-9135-x. Epub 2008 Mar 15. Biometals. 2008. PMID: 18344005
-
Gadolinium-containing MRI contrast agents: important variations on a theme for NSF.J Am Coll Radiol. 2008 Jan;5(1):29-35. doi: 10.1016/j.jacr.2007.08.014. J Am Coll Radiol. 2008. PMID: 18180006 Review.
Cited by
-
Chirality of New Drug Approvals (2013-2022): Trends and Perspectives.J Med Chem. 2024 Feb 22;67(4):2305-2320. doi: 10.1021/acs.jmedchem.3c02239. Epub 2024 Feb 12. J Med Chem. 2024. PMID: 38344815 Free PMC article. Review.
-
Experimentally Created Magnetic Force in Microbiological Space and On-Earth Studies: Perspectives and Restrictions.Cells. 2023 Jan 16;12(2):338. doi: 10.3390/cells12020338. Cells. 2023. PMID: 36672273 Free PMC article. Review.
References
-
- Balzer T., Bayer HealthCare Pharmaceuticals Inc. Medical Imaging Drugs Advisory Committee (MIDAC) U.S. Food & Drug Administration; Silver Spring, MD, USA: 2017. Presence of Gadolinium (Gd) in the brain and body.
-
- GE Healthcare Omniscan (Gadodiamide). U.S. Food and Drug Administration. [(accessed on 21 December 2021)];2010 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020123s037lbl.pdf.
-
- Bayer HealthCare Pharmaceuticals Gadavist (Gadobutrol). U.S. Food and Drug Administration. [(accessed on 21 December 2021)];2019 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/201277s017lbl.pdf.
-
- Lantheus Medical Imaging, Inc. Ablavar (Gadofosveset Trisodium). U.S. Food and Drug Administration. [(accessed on 21 December 2021)];2010 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021711s003lbl.pdf.
-
- Bayer HealthCare Pharmaceuticals Magnevist (Gadopentetate Dimeglumine). U.S. Food and Drug Administration. [(accessed on 21 December 2021)];2014 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/019596s057lbl.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


