Human Primary Olfactory Amygdala Subregions Form Distinct Functional Networks, Suggesting Distinct Olfactory Functions
- PMID: 34955769
- PMCID: PMC8695617
- DOI: 10.3389/fnsys.2021.752320
Human Primary Olfactory Amygdala Subregions Form Distinct Functional Networks, Suggesting Distinct Olfactory Functions
Abstract
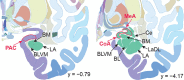
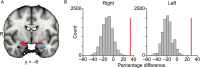
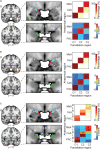
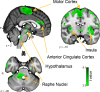
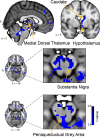
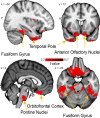
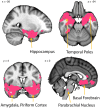
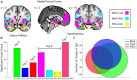
Three subregions of the amygdala receive monosynaptic projections from the olfactory bulb, making them part of the primary olfactory cortex. These primary olfactory areas are located at the anterior-medial aspect of the amygdala and include the medial amygdala (MeA), cortical amygdala (CoA), and the periamygdaloid complex (PAC). The vast majority of research on the amygdala has focused on the larger basolateral and basomedial subregions, which are known to be involved in implicit learning, threat responses, and emotion. Fewer studies have focused on the MeA, CoA, and PAC, with most conducted in rodents. Therefore, our understanding of the functions of these amygdala subregions is limited, particularly in humans. Here, we first conducted a review of existing literature on the MeA, CoA, and PAC. We then used resting-state fMRI and unbiased k-means clustering techniques to show that the anatomical boundaries of human MeA, CoA, and PAC accurately parcellate based on their whole-brain resting connectivity patterns alone, suggesting that their functional networks are distinct, relative both to each other and to the amygdala subregions that do not receive input from the olfactory bulb. Finally, considering that distinct functional networks are suggestive of distinct functions, we examined the whole-brain resting network of each subregion and speculated on potential roles that each region may play in olfactory processing. Based on these analyses, we speculate that the MeA could potentially be involved in the generation of rapid motor responses to olfactory stimuli (including fight/flight), particularly in approach/avoid contexts. The CoA could potentially be involved in olfactory-related reward processing, including learning and memory of approach/avoid responses. The PAC could potentially be involved in the multisensory integration of olfactory information with other sensory systems. These speculations can be used to form the basis of future studies aimed at clarifying the olfactory functions of these under-studied primary olfactory areas.
Keywords: amygdala; cortical amygdala; fMRI; medial amygdala; olfaction; periamygdaloid complex; resting connectivity.
Copyright © 2021 Noto, Zhou, Yang, Lane and Zelano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Afferent and efferent connections of the cortical and medial nuclei of the amygdala in sheep.J Chem Neuroanat. 2009 Mar;37(2):87-97. doi: 10.1016/j.jchemneu.2008.09.001. Epub 2008 Sep 12. J Chem Neuroanat. 2009. PMID: 18835351
-
Dendritic Organization of Olfactory Inputs to Medial Amygdala Neurons.J Neurosci. 2015 Sep 23;35(38):13020-8. doi: 10.1523/JNEUROSCI.0627-15.2015. J Neurosci. 2015. PMID: 26400933 Free PMC article.
-
Characterizing functional pathways of the human olfactory system.Elife. 2019 Jul 24;8:e47177. doi: 10.7554/eLife.47177. Elife. 2019. PMID: 31339489 Free PMC article.
-
Performance of a Computational Model of the Mammalian Olfactory System.In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 6. In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 6. PMID: 26042330 Free Books & Documents. Review.
-
Engineering Aspects of Olfaction.In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 1. In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 1. PMID: 26042329 Free Books & Documents. Review.
Cited by
-
The human social cognitive network contains multiple regions within the amygdala.Sci Adv. 2024 Nov 22;10(47):eadp0453. doi: 10.1126/sciadv.adp0453. Epub 2024 Nov 22. Sci Adv. 2024. PMID: 39576857 Free PMC article.
-
Olfactory Dysfunction in Parkinson's Disease, Its Functional and Neuroanatomical Correlates.NeuroSci. 2023 Jun 5;4(2):134-151. doi: 10.3390/neurosci4020013. eCollection 2023 Jun. NeuroSci. 2023. PMID: 39483318 Free PMC article. Review.
-
Monorhinal and birhinal odor processing in humans: an fMRI investigation.Chem Senses. 2024 Jan 1;49:bjae038. doi: 10.1093/chemse/bjae038. Chem Senses. 2024. PMID: 39387136
-
Structural Connectivity between Olfactory Tubercle and Ventrolateral Periaqueductal Gray Implicated in Human Feeding Behavior.J Neurosci. 2024 Jun 19;44(25):e2342232024. doi: 10.1523/JNEUROSCI.2342-23.2024. J Neurosci. 2024. PMID: 38755004
-
Social cognitive regions of human association cortex are selectively connected to the amygdala.bioRxiv [Preprint]. 2024 Jan 9:2023.12.06.570477. doi: 10.1101/2023.12.06.570477. bioRxiv. 2024. PMID: 38106046 Free PMC article. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


