The metabolic effects of multi-trace elements on parenteral nutrition for critically ill pediatric patients: a randomized controlled trial and metabolomic research
- PMID: 34765482
- PMCID: PMC8578764
- DOI: 10.21037/tp-21-456
The metabolic effects of multi-trace elements on parenteral nutrition for critically ill pediatric patients: a randomized controlled trial and metabolomic research
Abstract
Background: We investigated the efficacy and metabolic dose-effect of multi-trace element injection I [MTEI-(I)] for severe pediatric patients via a parallel, randomized control study.
Methods: The inclusion criteria were as follows: (I) patients who required parenteral nutrition (PN) due to various diseases, and were expected to receive PN for >5 days; (II) patients aged <18 years; (III) patients with no serious cardiac, hepatic, renal, or pulmonary dysfunction; and (IV) patients with an established central venous pathway. Enrolled patients were randomly assigned into two groups using sequentially numbered, sealed, opaque envelopes: Group A (low-dose group) received MTEI-(I) at 1 mL/kg/d, and Group B (high-dose group) received MTEI-(I) at 2 mL/kg/d, up to a maximum dose of 15 mL/d. The concentrations of manganese (Mn), copper (Cu), zinc (Zn), and selenium (Se) were detected. The following indexes were measured after 5 days of treatment (T5): β-oxidation of very-long-chain fatty acids, arginine and proline metabolism, pentose phosphate metabolism, ketone body metabolism, citric acid cycle, purine metabolism, caffeine metabolism, and pyruvate metabolism. The participants, care givers, and data analysis staff were blinded to the group assignment.
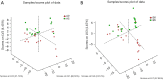
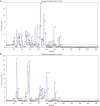
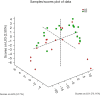
Results: Overall, at T5, Mn and Cu levels were decreased, while Zn and Se levels were increased. The increase of Zn levels (A: 0.170±0.479 vs. B: 0.193±0.900) and decrease of Cu levels (A: -0.240±0.382 vs. B: -0.373±0.465) of patients in Group B (n=22) were significantly higher than those in Group A (n=18). At T5, the β-oxidation of very-long-chain fatty acids, arginine and proline metabolism, pentose phosphate metabolism, ketone body metabolism, citric acid cycle, purine metabolism, caffeine metabolism, and pyruvate metabolism were variably decreased (P<0.05) in Group B compared to Group A.
Conclusions: Our results suggested that the high-dose administration of MTEI-(I) is safe for severe pediatric patients, and may alleviate inflammation and antioxidation, relieve hyperactivity caused by stress, and improve tissues-based hypoxia and renal function.
Trial registration: Chinese Clinical Trial Registry ChiCTR2100052198.
Keywords: Multi-trace element injection I [MTEI-(I)]; intensive care; metabolomics; parenteral nutrition (PN); pediatric.
2021 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-456). The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Assessing Selenium, Manganese, and Iodine Status in Pediatric Patients Receiving Parenteral Nutrition.Nutr Clin Pract. 2017 Aug;32(4):552-556. doi: 10.1177/0884533617698097. Epub 2017 Mar 14. Nutr Clin Pract. 2017. PMID: 28760114
-
Antioxidant trace elements serum levels in long-term parenteral nutrition (PN): Prevalence and infectious risk associated with deficiencies, a retrospective study from a tertiary home-PN center.Clin Nutr. 2017 Jun;36(3):812-817. doi: 10.1016/j.clnu.2016.05.008. Epub 2016 May 23. Clin Nutr. 2017. PMID: 27245643
-
Cross-sectional study: Relationship between serum trace elements and hypertension.J Trace Elem Med Biol. 2022 Jan;69:126893. doi: 10.1016/j.jtemb.2021.126893. Epub 2021 Nov 10. J Trace Elem Med Biol. 2022. PMID: 34798511
-
Role of trace elements in parenteral nutrition support of the surgical neonate.J Pediatr Surg. 2012 Apr;47(4):760-71. doi: 10.1016/j.jpedsurg.2012.01.015. J Pediatr Surg. 2012. PMID: 22498394 Review.
-
Status and potential diagnostic roles of essential trace elements in Kashin- Beck disease patients.J Trace Elem Med Biol. 2022 Jan;69:126880. doi: 10.1016/j.jtemb.2021.126880. Epub 2021 Oct 25. J Trace Elem Med Biol. 2022. PMID: 34717166 Review.
Cited by
-
Thiamine use is associated with better outcomes for traumatic brain injury patients.Front Nutr. 2024 Jul 5;11:1362817. doi: 10.3389/fnut.2024.1362817. eCollection 2024. Front Nutr. 2024. PMID: 39036489 Free PMC article.
References
-
- Pediatric Collaborative Group, Society of Parenteral and Enteral Nutrition, Chinese Medical Association . Guidelines for pediatric clinical application of enteral and parenteral nutritional support in China. Zhonghua Er Ke Za Zhi 2010;48:436-41. - PubMed
LinkOut - more resources
Full Text Sources

