Spontaneous Gait Transitions of Sprawling Quadruped Locomotion by Sensory-Driven Body-Limb Coordination Mechanisms
- PMID: 34393748
- PMCID: PMC8361603
- DOI: 10.3389/fnbot.2021.645731
Spontaneous Gait Transitions of Sprawling Quadruped Locomotion by Sensory-Driven Body-Limb Coordination Mechanisms
Abstract
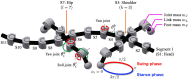
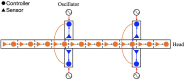
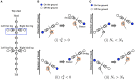
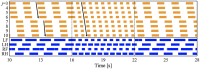
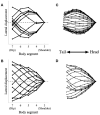
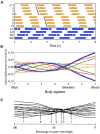
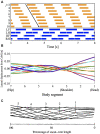
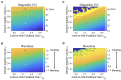
Deciphering how quadrupeds coordinate their legs and other body parts, such as the trunk, head, and tail (i.e., body-limb coordination), can provide informative insights to improve legged robot mobility. In this study, we focused on sprawling locomotion of the salamander and aimed to understand the body-limb coordination mechanisms through mathematical modeling and simulations. The salamander is an amphibian that moves on the ground by coordinating the four legs with lateral body bending. It uses standing and traveling waves of lateral bending that depend on the velocity and stepping gait. However, the body-limb coordination mechanisms responsible for this flexible gait transition remain elusive. This paper presents a central-pattern-generator-based model to reproduce spontaneous gait transitions, including changes in bending patterns. The proposed model implements four feedback rules (feedback from limb-to-limb, limb-to-body, body-to-limb, and body-to-body) without assuming any inter-oscillator coupling. The interplay of the feedback rules establishes a self-organized body-limb coordination that enables the reproduction of the speed-dependent gait transitions of salamanders, as well as various gait patterns observed in sprawling quadruped animals. This suggests that sensory feedback plays an essential role in flexible body-limb coordination during sprawling quadruped locomotion.
Keywords: body-limb coordination; decentralized control; gait transition; salamander locomotion; sensory feedback control.
Copyright © 2021 Suzuki, Kano, Ijspeert and Ishiguro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Decentralized control with cross-coupled sensory feedback between body and limbs in sprawling locomotion.Bioinspir Biomim. 2019 Sep 24;14(6):066010. doi: 10.1088/1748-3190/ab3ef6. Bioinspir Biomim. 2019. PMID: 31469116
-
Sprawling Quadruped Robot Driven by Decentralized Control With Cross-Coupled Sensory Feedback Between Legs and Trunk.Front Neurorobot. 2021 Jan 8;14:607455. doi: 10.3389/fnbot.2020.607455. eCollection 2020. Front Neurorobot. 2021. PMID: 33488377 Free PMC article.
-
A Quadruped Robot Exhibiting Spontaneous Gait Transitions from Walking to Trotting to Galloping.Sci Rep. 2017 Mar 21;7(1):277. doi: 10.1038/s41598-017-00348-9. Sci Rep. 2017. PMID: 28325917 Free PMC article.
-
Lateral undulation of the flexible spine of sprawling posture vertebrates.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018 Aug;204(8):707-719. doi: 10.1007/s00359-018-1275-z. Epub 2018 Jul 4. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018. PMID: 29974192 Review.
-
Decoding the mechanisms of gait generation in salamanders by combining neurobiology, modeling and robotics.Biol Cybern. 2013 Oct;107(5):545-64. doi: 10.1007/s00422-012-0543-1. Epub 2013 Feb 22. Biol Cybern. 2013. PMID: 23430277 Review.
Cited by
-
Editorial: Biological and Robotic Inter-Limb Coordination.Front Robot AI. 2022 Mar 22;9:875493. doi: 10.3389/frobt.2022.875493. eCollection 2022. Front Robot AI. 2022. PMID: 35391940 Free PMC article. No abstract available.
References
-
- Cartmill M., Lemelin P., Schmitt D. (2002). Support polygons and symmetrical gaits in mammals. Zool. J. Linnean Soc. 136, 401–420. 10.1046/j.1096-3642.2002.00038.x - DOI
LinkOut - more resources
Full Text Sources


