Run and hide: visual performance in a brittle star
- PMID: 34100540
- PMCID: PMC8214828
- DOI: 10.1242/jeb.236653
Run and hide: visual performance in a brittle star
Abstract
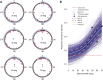
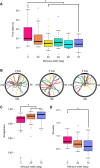
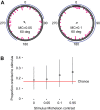
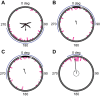
Spatial vision was recently reported in a brittle star, Ophiomastix wendtii, which lacks discrete eyes, but little is known about its visual ecology. Our aim was to better characterize the vision and visual ecology of this unusual visual system. We tested animal orientation relative to vertical bar stimuli at a range of angular widths and contrasts, to identify limits of angular and contrast detection. We also presented dynamic shadow stimuli, either looming towards or passing the animal overhead, to test for potential defensive responses. Finally, we presented animals lacking a single arm with a vertical bar stimulus known to elicit a response in intact animals. We found that O. wendtii orients to large (≥50 deg), high-contrast vertical bar stimuli, consistent with a shelter-seeking role and with photoreceptor acceptance angles estimated from morphology. We calculate poor optical sensitivity for individual photoreceptors, and predict dramatic oversampling for photoreceptor arrays. We also report responses to dark stimuli moving against a bright background - this is the first report of responses to moving stimuli in brittle stars and suggests additional defensive uses for vision in echinoderms. Finally, we found that animals missing a single arm orient less well to static stimuli, which requires further investigation.
Keywords: Echinoderms; Extraocular vision; Ophiuroids; Vision.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
Extraocular Vision in a Brittle Star Is Mediated by Chromatophore Movement in Response to Ambient Light.Curr Biol. 2020 Jan 20;30(2):319-327.e4. doi: 10.1016/j.cub.2019.11.042. Epub 2020 Jan 2. Curr Biol. 2020. PMID: 31902727
-
Visual Ecology: Now You See, Now You Don't.Curr Biol. 2020 Jan 20;30(2):R71-R73. doi: 10.1016/j.cub.2019.12.002. Curr Biol. 2020. PMID: 31962079
-
Whole-body photoreceptor networks are independent of 'lenses' in brittle stars.Proc Biol Sci. 2018 Jan 31;285(1871):20172590. doi: 10.1098/rspb.2017.2590. Proc Biol Sci. 2018. PMID: 29367398 Free PMC article.
-
Evidence for spatial vision in Chiton tuberculatus, a chiton with eyespots.J Exp Biol. 2018 Oct 1;221(Pt 19):jeb183632. doi: 10.1242/jeb.183632. J Exp Biol. 2018. PMID: 30127078
-
Neuroecology beyond the brain: learning in Echinodermata.Learn Behav. 2022 Mar;50(1):20-36. doi: 10.3758/s13420-021-00492-3. Epub 2021 Dec 7. Learn Behav. 2022. PMID: 34877627 Review.
Cited by
-
Looks like home: numerosity, but not spatial frequency guides preference in zebrafish larvae (Danio rerio).Anim Cogn. 2024 Jul 27;27(1):53. doi: 10.1007/s10071-024-01888-0. Anim Cogn. 2024. PMID: 39066805 Free PMC article.
References
-
- Aizenberg, J. and Hendler, G. (2004). Designing efficient microlens arrays: lessons from nature. J. Mater. Chem. 14, 2066. 10.1039/b402558j - DOI
-
- Batschelet, E. (1981). Circular Statistics in Biology. London: Academic Press Inc.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous


