Retargeting of NK-92 Cells against High-Risk Rhabdomyosarcomas by Means of an ERBB2 (HER2/Neu)-Specific Chimeric Antigen Receptor
- PMID: 33809981
- PMCID: PMC8004684
- DOI: 10.3390/cancers13061443
Retargeting of NK-92 Cells against High-Risk Rhabdomyosarcomas by Means of an ERBB2 (HER2/Neu)-Specific Chimeric Antigen Receptor
Abstract
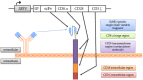
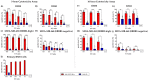
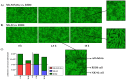
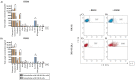
The dismal prognosis of pediatric and young adult patients with high-risk rhabdomyosarcoma (RMS) underscores the need for novel treatment options for this patient group. In previous studies, the tumor-associated surface antigen ERBB2 (HER2/neu) was identified as targetable in high-risk RMS. As a proof of concept, in this study, a novel treatment approach against RMS tumors using a genetically modified natural killer (NK)-92 cell line (NK-92/5.28.z) as an off-the-shelf ERBB2-chimeric antigen receptor (CAR)-engineered cell product was preclinically explored. In cytotoxicity assays, NK-92/5.28.z cells specifically recognized and efficiently eliminated RMS cell suspensions, tumor cell monolayers, and 3D tumor spheroids via the ERBB2-CAR even at effector-to-target ratios as low as 1:1. In contrast to unmodified parental NK-92 cells, which failed to lyse RMS cells, NK-92/5.28.z cells proliferated and became further activated through contact with ERBB2-positive tumor cells. Furthermore, high amounts of effector molecules, such as proinflammatory and antitumoral cytokines, were found in cocultures of NK-92/5.28.z cells with tumor cells. Taken together, our data suggest the enormous potential of this approach for improving the immunotherapy of treatment-resistant tumors, revealing the dual role of NK-92/5.28.z cells as CAR-targeted killers and modulators of endogenous adaptive immunity even in the inhibitory tumor microenvironment of high-risk RMS.
Keywords: CAR; ERBB2; HER2/neu; NK-92; RMS; cancer immunotherapy.
Conflict of interest statement
W.S.W. is named an inventor on patents and patent applications in the field of cancer immunotherapy owned by Georg-Speyer-Haus. The authors declare no other potential conflicts of interest.
Figures


Similar articles
-
Bortezomib promotes the TRAIL-mediated killing of resistant rhabdomyosarcoma by ErbB2/Her2-targeted CAR-NK-92 cells via DR5 upregulation.Mol Ther Oncol. 2024 Apr 11;32(2):200802. doi: 10.1016/j.omton.2024.200802. eCollection 2024 Jun 20. Mol Ther Oncol. 2024. PMID: 38706988 Free PMC article.
-
ErbB2 (HER2)-CAR-NK-92 cells for enhanced immunotherapy of metastatic fusion-driven alveolar rhabdomyosarcoma.Front Immunol. 2023 Aug 18;14:1228894. doi: 10.3389/fimmu.2023.1228894. eCollection 2023. Front Immunol. 2023. PMID: 37662907 Free PMC article.
-
ERBB2-CAR-Engineered Cytokine-Induced Killer Cells Exhibit Both CAR-Mediated and Innate Immunity Against High-Risk Rhabdomyosarcoma.Front Immunol. 2020 Oct 19;11:581468. doi: 10.3389/fimmu.2020.581468. eCollection 2020. Front Immunol. 2020. PMID: 33193388 Free PMC article.
-
CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy.Front Immunol. 2019 Nov 14;10:2683. doi: 10.3389/fimmu.2019.02683. eCollection 2019. Front Immunol. 2019. PMID: 31798595 Free PMC article. Review.
-
Reformation in chimeric antigen receptor based cancer immunotherapy: Redirecting natural killer cell.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):200-215. doi: 10.1016/j.bbcan.2018.01.005. Epub 2018 Jan 31. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29378229 Review.
Cited by
-
Natural killer cell-based cancer immunotherapy: from basics to clinical trials.Exp Hematol Oncol. 2024 Oct 16;13(1):101. doi: 10.1186/s40164-024-00561-z. Exp Hematol Oncol. 2024. PMID: 39415291 Free PMC article. Review.
-
Bortezomib promotes the TRAIL-mediated killing of resistant rhabdomyosarcoma by ErbB2/Her2-targeted CAR-NK-92 cells via DR5 upregulation.Mol Ther Oncol. 2024 Apr 11;32(2):200802. doi: 10.1016/j.omton.2024.200802. eCollection 2024 Jun 20. Mol Ther Oncol. 2024. PMID: 38706988 Free PMC article.
-
CAR NK Cell Therapy for the Treatment of Metastatic Melanoma: Potential & Prospects.Cells. 2023 Nov 30;12(23):2750. doi: 10.3390/cells12232750. Cells. 2023. PMID: 38067178 Free PMC article. Review.
-
ErbB2 (HER2)-CAR-NK-92 cells for enhanced immunotherapy of metastatic fusion-driven alveolar rhabdomyosarcoma.Front Immunol. 2023 Aug 18;14:1228894. doi: 10.3389/fimmu.2023.1228894. eCollection 2023. Front Immunol. 2023. PMID: 37662907 Free PMC article.
-
Natural Killer Cells: A Promising Kit in the Adoptive Cell Therapy Toolbox.Cancers (Basel). 2022 Nov 17;14(22):5657. doi: 10.3390/cancers14225657. Cancers (Basel). 2022. PMID: 36428748 Free PMC article. Review.
References
-
- Carli M., Colombatti R., Oberlin O., Bisogno G., Treuner J., Koscielniak E., Tridello G., Garaventa A., Pinkerton R., Stevens M. European Intergroup Studies (MMT4-89 and MMT4-91) on Childhood Metastatic Rhabdomyosarcoma: Final Results and Analysis of Prognostic Factors. J. Clin. Oncol. 2004;22:4787–4794. doi: 10.1200/JCO.2004.04.083. - DOI - PubMed
-
- Weigel B.J., Lyden E., Anderson J.R., Meyer W.H., Parham D.M., Rodeberg D.A., Michalski J.M., Hawkins D.S., Arndt C.A. Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2016;34:117–122. doi: 10.1200/JCO.2015.63.4048. - DOI - PMC - PubMed
-
- Gupta A.A., Chi Y.-Y., Anderson J.R., Lyden E., Weigel B., Arndt C., Meyer W.H., Rosenberg A., Hawkins U.S. Patterns of chemotherapy-induced toxicities and outcome in children and adolescents with metastatic rhabdomyosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer. 2017;64:e26479. doi: 10.1002/pbc.26479. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous


