Activated microglia drive demyelination via CSF1R signaling
- PMID: 33620118
- PMCID: PMC9250806
- DOI: 10.1002/glia.23980
Activated microglia drive demyelination via CSF1R signaling
Abstract
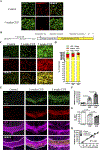
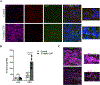
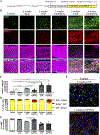
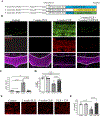
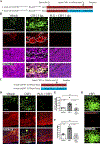
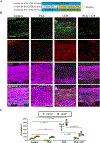
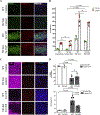
Microgliosis is a prominent pathological feature in many neurological diseases including multiple sclerosis (MS), a progressive auto-immune demyelinating disorder. The precise role of microglia, parenchymal central nervous system (CNS) macrophages, during demyelination, and the relative contributions of peripheral macrophages are incompletely understood. Classical markers used to identify microglia do not reliably discriminate between microglia and peripheral macrophages, confounding analyses. Here, we use a genetic fate mapping strategy to identify microglia as predominant responders and key effectors of demyelination in the cuprizone (CUP) model. Colony-stimulating factor 1 (CSF1), also known as macrophage colony-stimulating factor (M-CSF) - a secreted cytokine that regulates microglia development and survival-is upregulated in demyelinated white matter lesions. Depletion of microglia with the CSF1R inhibitor PLX3397 greatly abrogates the demyelination, loss of oligodendrocytes, and reactive astrocytosis that results from CUP treatment. Electron microscopy (EM) and serial block face imaging show myelin sheaths remain intact in CUP treated mice depleted of microglia. However, these CUP-damaged myelin sheaths are lost and robustly phagocytosed upon-repopulation of microglia. Direct injection of CSF1 into CNS white matter induces focal microgliosis and demyelination indicating active CSF1 signaling can promote demyelination. Finally, mice defective in adopting a toxic astrocyte phenotype that is driven by microglia nevertheless demyelinate normally upon CUP treatment implicating microglia rather than astrocytes as the primary drivers of CUP-mediated demyelination. Together, these studies indicate activated microglia are required for and can drive demyelination directly and implicate CSF1 signaling in these events.
Keywords: astrocyte; colony-stimulating factor 1; cuprizone; demyelination; microglia; myelin; oligodendrocyte.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Figures

Similar articles
-
Effect of the CSF1R inhibitor PLX3397 on remyelination of corpus callosum in a cuprizone-induced demyelination mouse model.J Cell Biochem. 2019 Jun;120(6):10576-10586. doi: 10.1002/jcb.28344. Epub 2019 Jan 10. J Cell Biochem. 2019. PMID: 30628737
-
Role of Macrophage Colony-Stimulating Factor Receptor on the Proliferation and Survival of Microglia Following Systemic Nerve and Cuprizone-Induced Injuries.Front Immunol. 2020 Jan 29;11:47. doi: 10.3389/fimmu.2020.00047. eCollection 2020. Front Immunol. 2020. PMID: 32082318 Free PMC article.
-
Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945.Acta Neuropathol Commun. 2018 Feb 15;6(1):9. doi: 10.1186/s40478-018-0510-8. Acta Neuropathol Commun. 2018. PMID: 29448957 Free PMC article.
-
Oligodendrocyte death and myelin loss in the cuprizone model: an updated overview of the intrinsic and extrinsic causes of cuprizone demyelination.Mol Neurodegener. 2022 May 7;17(1):34. doi: 10.1186/s13024-022-00538-8. Mol Neurodegener. 2022. PMID: 35526004 Free PMC article. Review.
-
Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?Neural Regen Res. 2023 Feb;18(2):267-272. doi: 10.4103/1673-5374.346538. Neural Regen Res. 2023. PMID: 35900401 Free PMC article. Review.
Cited by
-
The Hidden Hand in White Matter: Pericytes and the Puzzle of Demyelination.ACS Pharmacol Transl Sci. 2024 Sep 19;7(10):2912-2923. doi: 10.1021/acsptsci.4c00192. eCollection 2024 Oct 11. ACS Pharmacol Transl Sci. 2024. PMID: 39421660 Review.
-
Integrated multimodal cell atlas of Alzheimer's disease.Nat Neurosci. 2024 Dec;27(12):2366-2383. doi: 10.1038/s41593-024-01774-5. Epub 2024 Oct 14. Nat Neurosci. 2024. PMID: 39402379 Free PMC article.
-
Glial Cells as Key Regulators in Neuroinflammatory Mechanisms Associated with Multiple Sclerosis.Int J Mol Sci. 2024 Sep 4;25(17):9588. doi: 10.3390/ijms25179588. Int J Mol Sci. 2024. PMID: 39273535 Free PMC article. Review.
-
Deciphering glial contributions to CSF1R-related disorder via single-nuclear transcriptomic profiling: a case study.Acta Neuropathol Commun. 2024 Aug 28;12(1):139. doi: 10.1186/s40478-024-01853-5. Acta Neuropathol Commun. 2024. PMID: 39217398 Free PMC article.
-
Microglia specific alternative splicing alterations in multiple sclerosis.Aging (Albany NY). 2024 Aug 7;16(15):11656-11667. doi: 10.18632/aging.206045. Epub 2024 Aug 7. Aging (Albany NY). 2024. PMID: 39115871 Free PMC article.
References
-
- Ács P, & Komoly S (2012). Selective ultrastructural vulnerability in the cuprizone-induced experimental demyelination. Ideggyogyaszati Szemle, 65(7–8), 266–270. - PubMed
-
- Adams SJ, Kirk A, & Auer RN (2018). Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP): Integrating the literature on hereditary diffuse leukoencephalopathy with spheroids (HDLS) and pigmentary orthochromatic leukodystrophy (POLD). J Clin Neurosci, 48, 42–49. doi:10.1016/j.jocn.2017.10.060 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous


