Common fronto-temporal effective connectivity in humans and monkeys
- PMID: 33482086
- PMCID: PMC7927917
- DOI: 10.1016/j.neuron.2020.12.026
Common fronto-temporal effective connectivity in humans and monkeys
Abstract
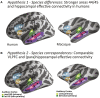
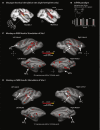
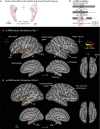
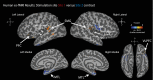
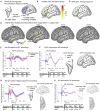
Human brain pathways supporting language and declarative memory are thought to have differentiated substantially during evolution. However, cross-species comparisons are missing on site-specific effective connectivity between regions important for cognition. We harnessed functional imaging to visualize the effects of direct electrical brain stimulation in macaque monkeys and human neurosurgery patients. We discovered comparable effective connectivity between caudal auditory cortex and both ventro-lateral prefrontal cortex (VLPFC, including area 44) and parahippocampal cortex in both species. Human-specific differences were clearest in the form of stronger hemispheric lateralization effects. In humans, electrical tractography revealed remarkably rapid evoked potentials in VLPFC following auditory cortex stimulation and speech sounds drove VLPFC, consistent with prior evidence in monkeys of direct auditory cortex projections to homologous vocalization-responsive regions. The results identify a common effective connectivity signature in human and nonhuman primates, which from auditory cortex appears equally direct to VLPFC and indirect to the hippocampus. VIDEO ABSTRACT.
Keywords: cognition; declarative memory; evolution; frontal cortex; hippocampus; language; neural principles; neuroimaging; neurophysiology.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Functional connection between posterior superior temporal gyrus and ventrolateral prefrontal cortex in human.Cereb Cortex. 2013 Oct;23(10):2309-21. doi: 10.1093/cercor/bhs220. Epub 2012 Aug 9. Cereb Cortex. 2013. PMID: 22879355 Free PMC article.
-
Evaluation of Structural Neural Connectivity Between the Primary Auditory Cortex and Cognition-Related Brain Areas Using Diffusion Tensor Tractography in 43 Normal Adults.Med Sci Monit. 2022 Feb 8;28:e936131. doi: 10.12659/MSM.936131. Med Sci Monit. 2022. PMID: 35132051 Free PMC article.
-
Mapping brain-wide excitatory projectome of primate prefrontal cortex at submicron resolution and comparison with diffusion tractography.Elife. 2022 May 20;11:e72534. doi: 10.7554/eLife.72534. Elife. 2022. PMID: 35593765 Free PMC article.
-
Direct and indirect parieto-medial temporal pathways for spatial navigation in humans: evidence from resting-state functional connectivity.Brain Struct Funct. 2017 May;222(4):1945-1957. doi: 10.1007/s00429-016-1318-6. Epub 2016 Oct 4. Brain Struct Funct. 2017. PMID: 27704218
-
Inactivation of Primate Prefrontal Cortex Impairs Auditory and Audiovisual Working Memory.J Neurosci. 2015 Jul 1;35(26):9666-75. doi: 10.1523/JNEUROSCI.1218-15.2015. J Neurosci. 2015. PMID: 26134649 Free PMC article.
Cited by
-
Spatiotemporal brain hierarchies of auditory memory recognition and predictive coding.Nat Commun. 2024 May 21;15(1):4313. doi: 10.1038/s41467-024-48302-4. Nat Commun. 2024. PMID: 38773109 Free PMC article.
-
Effects of transcranial magnetic stimulation on the human brain recorded with intracranial electrocorticography.Mol Psychiatry. 2024 May;29(5):1228-1240. doi: 10.1038/s41380-024-02405-y. Epub 2024 Feb 5. Mol Psychiatry. 2024. PMID: 38317012
-
Immediate neural impact and incomplete compensation after semantic hub disconnection.Nat Commun. 2023 Oct 7;14(1):6264. doi: 10.1038/s41467-023-42088-7. Nat Commun. 2023. PMID: 37805497 Free PMC article.
-
Failure to breathe persists without air hunger or alarm following amygdala seizures.JCI Insight. 2023 Nov 22;8(22):e172423. doi: 10.1172/jci.insight.172423. JCI Insight. 2023. PMID: 37788112 Free PMC article.
-
Update on Nonhuman Primate Models of Brain Disease and Related Research Tools.Biomedicines. 2023 Sep 12;11(9):2516. doi: 10.3390/biomedicines11092516. Biomedicines. 2023. PMID: 37760957 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


