International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function
- PMID: 33370241
- PMCID: PMC7770494
- DOI: 10.1124/pr.118.015552
International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function
Abstract
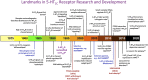
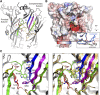
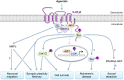
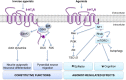
5-HT receptors expressed throughout the human body are targets for established therapeutics and various drugs in development. Their diversity of structure and function reflects the important role 5-HT receptors play in physiologic and pathophysiological processes. The present review offers a framework for the official receptor nomenclature and a detailed understanding of each of the 14 5-HT receptor subtypes, their roles in the systems of the body, and, where appropriate, the (potential) utility of therapeutics targeting these receptors. SIGNIFICANCE STATEMENT: This review provides a comprehensive account of the classification and function of 5-hydroxytryptamine receptors, including how they are targeted for therapeutic benefit.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures












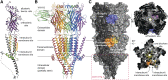

Similar articles
-
International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin).Pharmacol Rev. 1994 Jun;46(2):157-203. Pharmacol Rev. 1994. PMID: 7938165 Review.
-
Invertebrate serotonin receptors: a molecular perspective on classification and pharmacology.J Exp Biol. 2018 Oct 4;221(Pt 19):jeb184838. doi: 10.1242/jeb.184838. J Exp Biol. 2018. PMID: 30287590 Review.
-
International Union of Basic and Clinical Pharmacology. CXI. Pharmacology, Signaling, and Physiology of Metabotropic Glutamate Receptors.Pharmacol Rev. 2021 Jan;73(1):521-569. doi: 10.1124/pr.119.019133. Pharmacol Rev. 2021. PMID: 33361406 Review.
-
5-HT(6) receptor modulators: a patent update. Part 2. Diversity in heterocyclic scaffolds.Expert Opin Ther Pat. 2012 Oct;22(10):1123-68. doi: 10.1517/13543776.2012.722205. Epub 2012 Sep 7. Expert Opin Ther Pat. 2012. PMID: 22957857 Review.
-
International Union of Basic and Clinical Pharmacology CXIII: Nuclear Receptor Superfamily-Update 2023.Pharmacol Rev. 2023 Nov;75(6):1233-1318. doi: 10.1124/pharmrev.121.000436. Epub 2023 Aug 16. Pharmacol Rev. 2023. PMID: 37586884 Free PMC article. Review.
Cited by
-
Multi-step gene set analysis identified HTR3 family genes involving childhood acute lymphoblastic leukemia susceptibility.Arch Toxicol. 2024 Sep 25. doi: 10.1007/s00204-024-03881-5. Online ahead of print. Arch Toxicol. 2024. PMID: 39322821
-
Effect of Serotonin (5-Hydroxytryptamine) on Follicular Development in Porcine.Int J Mol Sci. 2024 Sep 4;25(17):9596. doi: 10.3390/ijms25179596. Int J Mol Sci. 2024. PMID: 39273540 Free PMC article.
-
Pharmacodynamic, pharmacokinetic and rat brain receptor occupancy profile of NLX-112, a highly selective 5-HT1A receptor biased agonist.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug 3. doi: 10.1007/s00210-024-03323-0. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39096379
-
Serotonin reuptake inhibitors improve muscle stem cell function and muscle regeneration in male mice.Nat Commun. 2024 Jul 31;15(1):6457. doi: 10.1038/s41467-024-50220-4. Nat Commun. 2024. PMID: 39085209 Free PMC article.
-
Uncovering the unique characteristics of different groups of 5-HT5AR ligands with reference to their interaction with the target protein.Pharmacol Rep. 2024 Oct;76(5):1130-1146. doi: 10.1007/s43440-024-00622-4. Epub 2024 Jul 6. Pharmacol Rep. 2024. PMID: 38971919 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

