Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study
- PMID: 33150319
- PMCID: PMC7599297
- DOI: 10.1016/j.eclinm.2020.100538
Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study
Abstract
Background: Psilocybin therapy has shown promise as a rapid-acting treatment for depression, anxiety, and demoralization in patients with serious medical illness (e.g., cancer) when paired with individual psychotherapy. This study assessed the safety and feasibility of psilocybin-assisted group therapy for demoralization in older long-term AIDS survivor (OLTAS) men, a population with a high degree of demoralization and traumatic loss.
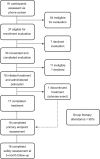
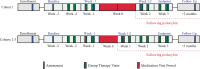
Methods: Self-identified gay men OLTAS with moderate-to-severe demoralization (Demoralization Scale-II ≥8) were recruited from the community of a major US city for a single-site open-label study of psilocybin-assisted group therapy comprising 8-10 group therapy visits and one psilocybin administration visit (0·3-0·36 mg/kg po). Primary outcomes were rate and severity of adverse events, and participant recruitment and retention. The primary clinical outcome was change in mean demoralization from baseline to end-of-treatment and to 3-month follow-up assessed with a two-way repeated measures ANOVA. Trial registration: Clinicaltrials.gov (NCT02950467).
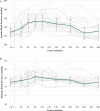
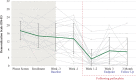
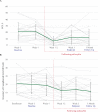
Findings: From 17 July 2017 to 16 January 2019, 18 participants (mean age 59·2 years (SD 4·4)) were enrolled, administered group therapy and psilocybin, and included in intent-to-treat analyses. We detected zero serious adverse reactions and two unexpected adverse reactions to psilocybin; seven participants experienced self-limited, severe expected adverse reactions. We detected a clinically meaningful change in demoralization from baseline to 3-month follow-up (mean difference -5·78 [SD 6·01], ηp 2 = 0·47, 90% CI 0·21-0·60).
Interpretation: We demonstrated the feasibility, relative safety, and potential efficacy of psilocybin-assisted group therapy for demoralization in OLTAS. Groups may be an effective and efficient means of delivering psychotherapy pre- and post-psilocybin to patients with complex medical and psychiatric needs.
Funding: Carey Turnbull, Heffter Research Institute, NIMH R25 MH060482, NIH UL1 TR001872, River Styx Foundation, Saisei Foundation, Sarlo Foundation, Stupski Foundation, Usona Institute, US Department of Veterans Affairs (Advanced Neurosciences Fellowship and IK2CX001495).
Keywords: Demoralization; HIV/AIDS; Long-term survivors; Psilocybin; Trauma.
© 2020 The Authors.
Conflict of interest statement
The authors declare no financial conflicts of interest related to for-profit entities. The following grants supported work on this trial: 10.13039/100000025NIMH R25 MH060482, 10.13039/100000009NIH UL1 TR001872, US Department of Veterans Affairs (Advanced Neurosciences Fellowship and IK2CX001495). BA and JW received philanthropic gifts from the following individuals and non-profit organizations to support this trial: Carey Turnbull, Heffter Research Institute, River Styx Foundation, Saisei Foundation, Sarlo Foundation, Stupski Foundation, Usona Institute. JW is conducting a trial sponsored by the non-profit Usona Institute. MB receives consulting fees from Beneath the Surface Foundation.
Figures
Similar articles
-
Psilocybin-assisted group therapy in patients with cancer diagnosed with a major depressive disorder.Cancer. 2024 Apr 1;130(7):1137-1146. doi: 10.1002/cncr.35010. Epub 2023 Dec 18. Cancer. 2024. PMID: 38105655 Clinical Trial.
-
Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer.J Psychopharmacol. 2020 Feb;34(2):155-166. doi: 10.1177/0269881119897615. Epub 2020 Jan 9. J Psychopharmacol. 2020. PMID: 31916890
-
Psilocybin-Assisted Group Therapy and Attachment: Observed Reduction in Attachment Anxiety and Influences of Attachment Insecurity on the Psilocybin Experience.ACS Pharmacol Transl Sci. 2020 Dec 9;4(2):526-532. doi: 10.1021/acsptsci.0c00169. eCollection 2021 Apr 9. ACS Pharmacol Transl Sci. 2020. PMID: 33860182 Free PMC article.
-
Psilocybin for Trauma-Related Disorders.Curr Top Behav Neurosci. 2022;56:319-332. doi: 10.1007/7854_2022_366. Curr Top Behav Neurosci. 2022. PMID: 35711024 Review.
-
Systematic Review of Interventions for Demoralization in Patients With Cancer.J Nerv Ment Dis. 2023 Apr 1;211(4):314-326. doi: 10.1097/NMD.0000000000001615. J Nerv Ment Dis. 2023. PMID: 36975545 Review.
Cited by
-
The conceptual framework for the therapeutic approach used in phase 3 trials of MDMA-assisted therapy for PTSD.Front Psychol. 2024 Nov 4;15:1427531. doi: 10.3389/fpsyg.2024.1427531. eCollection 2024. Front Psychol. 2024. PMID: 39559692 Free PMC article.
-
Adverse Events in Studies of Classic Psychedelics: A Systematic Review and Meta-Analysis.JAMA Psychiatry. 2024 Dec 1;81(12):1225-1235. doi: 10.1001/jamapsychiatry.2024.2546. JAMA Psychiatry. 2024. PMID: 39230883
-
Psilocybin-assisted therapy and HIV-related shame.Sci Rep. 2024 Aug 2;14(1):17919. doi: 10.1038/s41598-024-68908-4. Sci Rep. 2024. PMID: 39095420 Free PMC article.
-
Psilocybin for the treatment of Alzheimer's disease.Front Neurosci. 2024 Jul 10;18:1420601. doi: 10.3389/fnins.2024.1420601. eCollection 2024. Front Neurosci. 2024. PMID: 39050672 Free PMC article. Review.
-
Cognitive functioning associated with acute and subacute effects of classic psychedelics and MDMA - a systematic review and meta-analysis.Sci Rep. 2024 Jun 26;14(1):14782. doi: 10.1038/s41598-024-65391-9. Sci Rep. 2024. PMID: 38926480 Free PMC article.
References
-
- Robinson S., Kissane D.W., Brooker J., Burney S. A systematic review of the demoralization syndrome in individuals with progressive disease and cancer: a decade of research. J Pain Symptom Manag. 2015;49:595–610. - PubMed
-
- Julião M., Nunes B., Barbosa A. Prevalence and factors associated with demoralization syndrome in patients with advanced disease: results from a cross-sectional Portuguese study. Palliat Support Care. 2016;14(5):468–473. - PubMed
-
- Ignatius J., De La Garza R., II Frequency of demoralization and depression in cancer patients. Gen Hosp Psychiatry. 2019;60(Sep –Oct):137–140. - PubMed
-
- Nanni M.G., Caruso R., Travado L., Ventura C., Palma A., Berardi A.M. Relationship of demoralization with anxiety, depression, and quality of life: a Southern European study of Italian and Portuguese cancer patients. Psycho‐Oncol. 2018;27(11):2616–2622. - PubMed
-
- Robinson S., Kissane D.W., Brooker J., Hempton C., Michael N., Fischer J. Refinement and revalidation of the demoralization scale: the DS‐II—External validity. Cancer. 2016;122(14):2260–2267. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources


