Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia
- PMID: 32896291
- PMCID: PMC7471804
- DOI: 10.1016/S0140-6736(20)31866-3
Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia
Erratum in
-
Department of Error.Lancet. 2021 Jan 9;397(10269):98. doi: 10.1016/S0140-6736(20)32721-5. Lancet. 2021. PMID: 33422261 Free PMC article. No abstract available.
Abstract
Background: We developed a heterologous COVID-19 vaccine consisting of two components, a recombinant adenovirus type 26 (rAd26) vector and a recombinant adenovirus type 5 (rAd5) vector, both carrying the gene for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein (rAd26-S and rAd5-S). We aimed to assess the safety and immunogenicity of two formulations (frozen and lyophilised) of this vaccine.
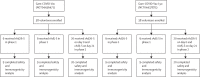
Methods: We did two open, non-randomised phase 1/2 studies at two hospitals in Russia. We enrolled healthy adult volunteers (men and women) aged 18-60 years to both studies. In phase 1 of each study, we administered intramuscularly on day 0 either one dose of rAd26-S or one dose of rAd5-S and assessed the safety of the two components for 28 days. In phase 2 of the study, which began no earlier than 5 days after phase 1 vaccination, we administered intramuscularly a prime-boost vaccination, with rAd26-S given on day 0 and rAd5-S on day 21. Primary outcome measures were antigen-specific humoral immunity (SARS-CoV-2-specific antibodies measured by ELISA on days 0, 14, 21, 28, and 42) and safety (number of participants with adverse events monitored throughout the study). Secondary outcome measures were antigen-specific cellular immunity (T-cell responses and interferon-γ concentration) and change in neutralising antibodies (detected with a SARS-CoV-2 neutralisation assay). These trials are registered with ClinicalTrials.gov, NCT04436471 and NCT04437875.
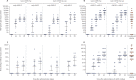
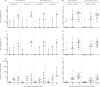
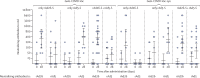
Findings: Between June 18 and Aug 3, 2020, we enrolled 76 participants to the two studies (38 in each study). In each study, nine volunteers received rAd26-S in phase 1, nine received rAd5-S in phase 1, and 20 received rAd26-S and rAd5-S in phase 2. Both vaccine formulations were safe and well tolerated. The most common adverse events were pain at injection site (44 [58%]), hyperthermia (38 [50%]), headache (32 [42%]), asthenia (21 [28%]), and muscle and joint pain (18 [24%]). Most adverse events were mild and no serious adverse events were detected. All participants produced antibodies to SARS-CoV-2 glycoprotein. At day 42, receptor binding domain-specific IgG titres were 14 703 with the frozen formulation and 11 143 with the lyophilised formulation, and neutralising antibodies were 49·25 with the frozen formulation and 45·95 with the lyophilised formulation, with a seroconversion rate of 100%. Cell-mediated responses were detected in all participants at day 28, with median cell proliferation of 2·5% CD4+ and 1·3% CD8+ with the frozen formulation, and a median cell proliferation of 1·3% CD4+ and 1·1% CD8+ with the lyophilised formulation.
Interpretation: The heterologous rAd26 and rAd5 vector-based COVID-19 vaccine has a good safety profile and induced strong humoral and cellular immune responses in participants. Further investigation is needed of the effectiveness of this vaccine for prevention of COVID-19.
Funding: Ministry of Health of the Russian Federation.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
COVID-19 vaccines: early success and remaining challenges.Lancet. 2020 Sep 26;396(10255):868-869. doi: 10.1016/S0140-6736(20)31867-5. Epub 2020 Sep 4. Lancet. 2020. PMID: 32896290 Free PMC article. No abstract available.
-
Safety and efficacy of the Russian COVID-19 vaccine: more information needed.Lancet. 2020 Oct 3;396(10256):e53. doi: 10.1016/S0140-6736(20)31960-7. Epub 2020 Sep 21. Lancet. 2020. PMID: 32971041 Free PMC article. No abstract available.
-
Safety and efficacy of the Russian COVID-19 vaccine: more information needed - Authors' reply.Lancet. 2020 Oct 3;396(10256):e54-e55. doi: 10.1016/S0140-6736(20)31970-X. Epub 2020 Sep 21. Lancet. 2020. PMID: 32971043 Free PMC article. No abstract available.
-
Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial.Lancet. 2021 May 22;397(10288):1881-1883. doi: 10.1016/S0140-6736(21)00899-0. Epub 2021 May 12. Lancet. 2021. PMID: 33991475 Free PMC article. No abstract available.
Similar articles
-
Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial.Lancet. 2020 Aug 15;396(10249):467-478. doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Lancet. 2020. PMID: 32702298 Free PMC article. Clinical Trial.
-
Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial.Lancet. 2020 Jun 13;395(10240):1845-1854. doi: 10.1016/S0140-6736(20)31208-3. Epub 2020 May 22. Lancet. 2020. PMID: 32450106 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet. 2020 Aug 15;396(10249):479-488. doi: 10.1016/S0140-6736(20)31605-6. Epub 2020 Jul 20. Lancet. 2020. PMID: 32702299 Free PMC article. Clinical Trial.
-
COVID-19 Vaccines: "Warp Speed" Needs Mind Melds, Not Warped Minds.J Virol. 2020 Aug 17;94(17):e01083-20. doi: 10.1128/JVI.01083-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32591466 Free PMC article. Review.
-
Effects of MS disease-modifying therapies on responses to vaccinations: A review.Mult Scler Relat Disord. 2020 Oct;45:102439. doi: 10.1016/j.msard.2020.102439. Epub 2020 Aug 1. Mult Scler Relat Disord. 2020. PMID: 32769063 Free PMC article. Review.
Cited by
-
Effect of COVID-19 vaccine in adults infected with the Delta variant of SARS-CoV-2: a retrospective cohort study.J Thorac Dis. 2024 Oct 31;16(10):6983-6998. doi: 10.21037/jtd-24-1351. Epub 2024 Oct 15. J Thorac Dis. 2024. PMID: 39552877 Free PMC article.
-
Safety, tolerability, and immunogenicity of PIKA-adjuvanted recombinant SARS-CoV-2 spike protein subunit vaccine in healthy adults: an open-label randomized phase I clinical trial.Clin Exp Vaccine Res. 2024 Oct;13(4):315-328. doi: 10.7774/cevr.2024.13.4.315. Epub 2024 Oct 31. Clin Exp Vaccine Res. 2024. PMID: 39525677 Free PMC article.
-
Humoral and cellular immune response to AZD1222 /Covishield and BV152/Covaxin COVID-19 vaccines among adults in India.Hum Vaccin Immunother. 2024 Dec 31;20(1):2410579. doi: 10.1080/21645515.2024.2410579. Epub 2024 Oct 21. Hum Vaccin Immunother. 2024. PMID: 39434214 Free PMC article.
-
In Silico Design of a Trans-Amplifying RNA-Based Vaccine against SARS-CoV-2 Structural Proteins.Adv Virol. 2024 Sep 30;2024:3418062. doi: 10.1155/2024/3418062. eCollection 2024. Adv Virol. 2024. PMID: 39380944 Free PMC article.
-
The BCG vaccine and SARS-CoV-2: Could there be a beneficial relationship?Heliyon. 2024 Sep 19;10(18):e38085. doi: 10.1016/j.heliyon.2024.e38085. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39347386 Free PMC article. Review.
References
-
- WHO Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 - PubMed
-
- WHO WHO Director-General's opening remarks at the media briefing on COVID-19. March 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- WHO Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief. July 9, 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-c...
-
- WHO Draft landscape of COVID-19 candidate vaccines. Aug 28, 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous


