A review of the newly identified impurity profiles in methamphetamine seizures
- PMID: 32637907
- PMCID: PMC7327898
- DOI: 10.1016/j.fsisyn.2020.06.004
A review of the newly identified impurity profiles in methamphetamine seizures
Abstract
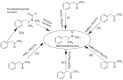
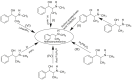
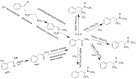
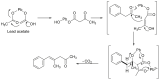
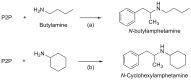
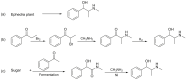
Forensic intelligence of synthetic illicit drugs suffers a problem of continuous introduction of new synthetic methods, modification of the existing routes of manufacture, and adulterations practiced by criminal networks. Impurity profiling has been indispensable in methamphetamine intelligence based on precursors, synthetic routes, and chemical modifications during trafficking. Law enforcement authorities maintain the credibility and integrity of intelligence information through constant monitoring of the chemical signatures in the illicit drug market. Changes in the synthetic pattern result in new impurity profiles that are important in keeping valuable intelligence information on clandestine laboratories, new synthetic routes, trafficking patterns, and geographical sources of illicit Methamphetamine. This review presents a critical analysis of the methamphetamine impurity profiles and more specifically, profiling based on impurity profiles from Leuckart, Reductive amination, Moscow, Emde, Nagai, Birch, Moscow route; a recent nitrostyrene route and stable isotope signatures. It also highlights the discrimination of ephedrine from pseudoephedrine sources and the emerging methamphetamine profiling based on stable isotopes.
Keywords: Impurity profiles; Methamphetamine; Stable isotopes; Synthetic route.
© 2020 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
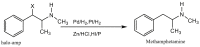
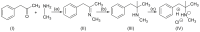
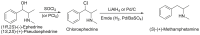
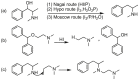
Similar articles
-
Characterization of route specific impurities found in methamphetamine synthesized by the Leuckart and reductive amination methods.Anal Chem. 2009 Sep 1;81(17):7342-8. doi: 10.1021/ac9005588. Anal Chem. 2009. PMID: 19637924 Free PMC article.
-
A review of impurity profiling and synthetic route of manufacture of methylamphetamine, 3,4-methylenedioxymethylamphetamine, amphetamine, dimethylamphetamine and p-methoxyamphetamine.Forensic Sci Int. 2013 Jan 10;224(1-3):8-26. doi: 10.1016/j.forsciint.2012.10.040. Epub 2012 Nov 24. Forensic Sci Int. 2013. PMID: 23182870
-
Investigation by direct-infusion ESI-MS and GC-MS of an alleged Leuckart route-specific impurity of methamphetamine.Forensic Sci Int. 2018 Jul;288:278-282. doi: 10.1016/j.forsciint.2018.05.007. Epub 2018 May 16. Forensic Sci Int. 2018. PMID: 29787972
-
An evolving problem: methamphetamine production and trafficking in the United States.Int J Drug Policy. 2012 Nov;23(6):426-35. doi: 10.1016/j.drugpo.2012.07.004. Epub 2012 Sep 1. Int J Drug Policy. 2012. PMID: 22943831 Review.
-
Synthetic origin of illicit methylamphetamine in Australia: 2011-2020.Drug Test Anal. 2022 Mar;14(3):427-438. doi: 10.1002/dta.3117. Epub 2021 Jul 7. Drug Test Anal. 2022. PMID: 34156767 Review.
Cited by
-
75 years of forensic profiling: A critical review.Heliyon. 2024 Oct 18;10(20):e39490. doi: 10.1016/j.heliyon.2024.e39490. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39506939 Free PMC article. Review.
-
Pharmacological Treatments for Methamphetamine Use Disorder: Current Status and Future Targets.Subst Abuse Rehabil. 2024 Aug 30;15:125-161. doi: 10.2147/SAR.S431273. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39228432 Free PMC article. Review.
-
Changes in drug availability patterns on Tanzanian mainland: The effects of the surge operations deterrent strategy.Forensic Sci Int Synerg. 2022 Nov 30;5:100295. doi: 10.1016/j.fsisyn.2022.100295. eCollection 2022. Forensic Sci Int Synerg. 2022. PMID: 36479426 Free PMC article.
-
Trends in illicit drugs based on the analysis of seizures from the Tanzania mainland drugs market.Forensic Sci Int Synerg. 2021 Nov 18;3:100209. doi: 10.1016/j.fsisyn.2021.100209. eCollection 2021. Forensic Sci Int Synerg. 2021. PMID: 34849480 Free PMC article.
References
-
- UN United Nations treaty collection: United Nations convention against illicit traffic in narcotic drugs and psychotropic substances. 1988;1–37
-
- INCB . sixth ed. Vienna International Centre; Vienna: 2018. International Narcotics Control Board: List of Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances under International Control.
-
- UNODC . vol. 156. United Nations Office on Drugs and Crime; 2016. (World Drug Report).
-
- DEA, “Drug Enforcement Administration, Methamphetamine,” Divers. Control Div Drug Chem. Eval. Sect. 2020:4250.
-
- UNODC . United Nations Office on Drugs and Crime; Vienna: 2019. World Drug Report.
Publication types
LinkOut - more resources
Full Text Sources


