GBA1 mutations: Prospects for exosomal biomarkers in α-synuclein pathologies
- PMID: 31761523
- PMCID: PMC7002237
- DOI: 10.1016/j.ymgme.2019.10.006
GBA1 mutations: Prospects for exosomal biomarkers in α-synuclein pathologies
Abstract
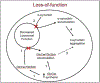
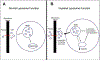
The discovery that patients with Gaucher Disease (GD), a rare lysosomal storage disorder, were developing symptoms similar to Parkinson's disease (PD) led to investigation of the relationship between the two seemingly unrelated pathologies. GD, an autosomal recessive disorder, is the result of a biallelic mutation in the gene GBA1, which encodes for the enzyme glucocerebrosidase (GCase). Since the observation of its relation to PD, GBA1 mutations have become recognized as the most common genetic risk factor for development of synucleinopathies such as PD and dementia with Lewy bodies. Although the exact mechanism by which GBA1 mutations promote PD is unknown, current understanding suggests that impaired GCase inhibits lysosomal activity and decreases the overall ability of the cell to degrade proteins, specifically the neuronal protein α-synuclein. Decreased elimination of α-synuclein can lead to its abnormal accumulation and aggregation, an important component of PD development. Further understanding of how decreased GCase activity increases risk for α-synuclein pathology can assist with the development of clinical biomarkers for early detection of synucleinopathies, as well as promote novel treatments tailored for people with a GBA1 mutation. Historically, α-synuclein has not been a reliable biomarker for PD. However, recent research on α-synuclein content within exosomes, which are small vesicles released by cells that carry specific cellular cargo, has yielded encouraging results. Moreover, decreased GCase activity has been shown to influence exosomal contents. Exosomes have emerged as a promising new avenue for the identification of novel biomarkers and therapeutic targets aimed at improving neuronal GCase function and limiting the development of synucleinopathies.
Keywords: Alpha-synuclein; Biomarker; Carriers; Exosomes; GBA1; Gaucher disease.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest PJ declare no conflict of interest. RVK have received grants from NIH, Pfizer Inc. and Sanofi-Genzyme outside of this work. JCC have received grants from NIH, Pfizer Inc. and Sanofi-Genzyme outside of this work. PJT has received grants from NIH, Parkinson Study Group, Northwestern University, Biogen Inc., and Bristol-Myers Squibb. NJW has received grants from Sanofi-Genzyme and Takeda-Shire, personal fees from Sanofi-Genzyme, Takeda-Shire and Pfizer Inc., and non-financial support from Sanofi-Genzyme.
Figures

Similar articles
-
Lysosomal functions and dysfunctions: Molecular and cellular mechanisms underlying Gaucher disease and its association with Parkinson disease.Adv Drug Deliv Rev. 2022 Aug;187:114402. doi: 10.1016/j.addr.2022.114402. Epub 2022 Jun 25. Adv Drug Deliv Rev. 2022. PMID: 35764179 Review.
-
Glucocerebrosidase mutations and synucleinopathies: Toward a model of precision medicine.Mov Disord. 2019 Jan;34(1):9-21. doi: 10.1002/mds.27583. Epub 2018 Dec 27. Mov Disord. 2019. PMID: 30589955 Review.
-
The relationship between glucocerebrosidase mutations and Parkinson disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):77-90. doi: 10.1111/jnc.13385. Epub 2016 Feb 10. J Neurochem. 2016. PMID: 26860875 Free PMC article. Review.
-
GBA1 Gene Mutations in α-Synucleinopathies-Molecular Mechanisms Underlying Pathology and Their Clinical Significance.Int J Mol Sci. 2023 Jan 20;24(3):2044. doi: 10.3390/ijms24032044. Int J Mol Sci. 2023. PMID: 36768367 Free PMC article. Review.
-
Acid ceramidase inhibition ameliorates α-synuclein accumulation upon loss of GBA1 function.Hum Mol Genet. 2018 Jun 1;27(11):1972-1988. doi: 10.1093/hmg/ddy105. Hum Mol Genet. 2018. PMID: 29579237 Free PMC article.
Cited by
-
Lipids and α-Synuclein: adding further variables to the equation.Front Mol Biosci. 2024 Aug 12;11:1455817. doi: 10.3389/fmolb.2024.1455817. eCollection 2024. Front Mol Biosci. 2024. PMID: 39188788 Free PMC article. Review.
-
More than meets the eye in Parkinson's disease and other synucleinopathies: from proteinopathy to lipidopathy.Acta Neuropathol. 2023 Sep;146(3):369-385. doi: 10.1007/s00401-023-02601-0. Epub 2023 Jul 8. Acta Neuropathol. 2023. PMID: 37421475 Free PMC article. Review.
-
β-Glucocerebrosidase Deficiency Activates an Aberrant Lysosome-Plasma Membrane Axis Responsible for the Onset of Neurodegeneration.Cells. 2022 Jul 29;11(15):2343. doi: 10.3390/cells11152343. Cells. 2022. PMID: 35954187 Free PMC article.
-
Contribution of Autophagy-Lysosomal Pathway in the Exosomal Secretion of Alpha-Synuclein and Its Impact in the Progression of Parkinson's Disease.Front Mol Neurosci. 2022 Feb 17;15:805087. doi: 10.3389/fnmol.2022.805087. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35250476 Free PMC article. Review.
-
Gaucher disease - more than just a rare lipid storage disease.J Mol Med (Berl). 2022 Apr;100(4):499-518. doi: 10.1007/s00109-021-02174-z. Epub 2022 Jan 23. J Mol Med (Berl). 2022. PMID: 35066608 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


