Hybrid Cyanobacterial-Tobacco Rubisco Supports Autotrophic Growth and Procarboxysomal Aggregation
- PMID: 31744936
- PMCID: PMC6997680
- DOI: 10.1104/pp.19.01193
Hybrid Cyanobacterial-Tobacco Rubisco Supports Autotrophic Growth and Procarboxysomal Aggregation
Abstract
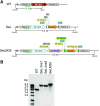
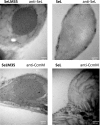
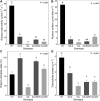
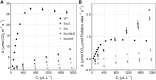
Much of the research aimed at improving photosynthesis and crop productivity attempts to overcome shortcomings of the primary CO2-fixing enzyme Rubisco. Cyanobacteria utilize a CO2-concentrating mechanism (CCM), which encapsulates Rubisco with poor specificity but a relatively fast catalytic rate within a carboxysome microcompartment. Alongside the active transport of bicarbonate into the cell and localization of carbonic anhydrase within the carboxysome shell with Rubisco, cyanobacteria are able to overcome the limitations of Rubisco via localization within a high-CO2 environment. As part of ongoing efforts to engineer a β-cyanobacterial CCM into land plants, we investigated the potential for Rubisco large subunits (LSU) from the β-cyanobacterium Synechococcus elongatus (Se) to form aggregated Rubisco complexes with the carboxysome linker protein CcmM35 within tobacco (Nicotiana tabacum) chloroplasts. Transplastomic plants were produced that lacked cognate Se Rubisco small subunits (SSU) and expressed the Se LSU in place of tobacco LSU, with and without CcmM35. Plants were able to form a hybrid enzyme utilizing tobacco SSU and the Se LSU, allowing slow autotrophic growth in high CO2 CcmM35 was able to form large Rubisco aggregates with the Se LSU, and these incorporated small amounts of native tobacco SSU. Plants lacking the Se SSU showed delayed growth, poor photosynthetic capacity, and significantly reduced Rubisco activity compared with both wild-type tobacco and lines expressing the Se SSU. These results demonstrate the ability of the Se LSU and CcmM35 to form large aggregates without the cognate Se SSU in planta, harboring active Rubisco that enables plant growth, albeit at a much slower pace than plants expressing the cognate Se SSU.
© 2020 The authors. All Rights Reserved.
Figures


Similar articles
-
Towards engineering carboxysomes into C3 plants.Plant J. 2016 Jul;87(1):38-50. doi: 10.1111/tpj.13139. Epub 2016 Jun 20. Plant J. 2016. PMID: 26867858 Free PMC article.
-
Transgenic tobacco plants with improved cyanobacterial Rubisco expression but no extra assembly factors grow at near wild-type rates if provided with elevated CO2.Plant J. 2016 Jan;85(1):148-60. doi: 10.1111/tpj.13098. Plant J. 2016. PMID: 26662726 Free PMC article.
-
A faster Rubisco with potential to increase photosynthesis in crops.Nature. 2014 Sep 25;513(7519):547-50. doi: 10.1038/nature13776. Epub 2014 Sep 17. Nature. 2014. PMID: 25231869 Free PMC article.
-
A carboxysome-based CO2 concentrating mechanism for C3 crop chloroplasts: advances and the road ahead.Plant J. 2024 May;118(4):940-952. doi: 10.1111/tpj.16667. Epub 2024 Feb 6. Plant J. 2024. PMID: 38321620 Review.
-
Improving photosynthesis through the enhancement of Rubisco carboxylation capacity.Biochem Soc Trans. 2021 Nov 1;49(5):2007-2019. doi: 10.1042/BST20201056. Biochem Soc Trans. 2021. PMID: 34623388 Review.
Cited by
-
Discovery of a readily heterologously expressed Rubisco from the deep sea with potential for CO2 capture.Bioresour Bioprocess. 2021 Sep 7;8(1):86. doi: 10.1186/s40643-021-00439-6. Bioresour Bioprocess. 2021. PMID: 38650243 Free PMC article.
-
Research Progress in Improving Photosynthetic Efficiency.Int J Mol Sci. 2023 May 26;24(11):9286. doi: 10.3390/ijms24119286. Int J Mol Sci. 2023. PMID: 37298238 Free PMC article. Review.
-
Engineering α-carboxysomes into plant chloroplasts to support autotrophic photosynthesis.Nat Commun. 2023 Apr 25;14(1):2118. doi: 10.1038/s41467-023-37490-0. Nat Commun. 2023. PMID: 37185249 Free PMC article.
-
Producing fast and active Rubisco in tobacco to enhance photosynthesis.Plant Cell. 2023 Feb 20;35(2):795-807. doi: 10.1093/plcell/koac348. Plant Cell. 2023. PMID: 36471570 Free PMC article.
-
Structural insights into cyanobacterial RuBisCO assembly coordinated by two chaperones Raf1 and RbcX.Cell Discov. 2022 Sep 20;8(1):93. doi: 10.1038/s41421-022-00436-9. Cell Discov. 2022. PMID: 36123352 Free PMC article.
References
-
- Andrews TJ. (1988) Catalysis by cyanobacterial ribulose-bisphosphate carboxylase large subunits in the complete absence of small subunits. J Biol Chem 263: 12213–12219 - PubMed
-
- Andrews TJ, Lorimer GH (1985) Catalytic properties of a hybrid between cyanobacterial large subunits and higher plant small subunits of ribulose bisphosphate carboxylase-oxygenase. J Biol Chem 260: 4632–4636 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


