Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera
- PMID: 31731445
- PMCID: PMC6921062
- DOI: 10.3390/insects10110371
Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera
Abstract
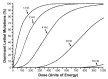
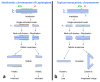
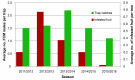
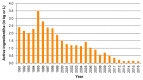
Over the past 30 years, the sterile insect technique (SIT) has become a regular component of area-wide integrated pest management (AW-IPM) programs against several major agricultural pests and vectors of severe diseases. The SIT-based programs have been especially successful against dipteran pests. However, the SIT applicability for controlling lepidopteran pests has been challenging, mainly due to their high resistance to the ionizing radiation that is used to induce sterility. Nevertheless, the results of extensive research and currently operating SIT programs show that most problems with the implementation of SIT against pest Lepidoptera have been successfully resolved. Here, we summarize the cytogenetic peculiarities of Lepidoptera that should be considered in the development and application of SIT for a particular pest species. We also discuss the high resistance of Lepidoptera to ionizing radiation, and present the principle of derived technology based on inherited sterility (IS). Furthermore, we present successful SIT/IS applications against five major lepidopteran pests, and summarize the results of research on the quality control of reared and released insects, which is of great importance for their field performance. In the light of new research findings, we also discuss options for the development of genetic sexing strains, which is a challenge to further improve the applicability of SIT/IS against selected lepidopteran pests.
Keywords: SIT; cytogenetics; genetic sexing; inherited sterility; moths; pest control programs; quality control.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the interpretation of data, in the writing of the manuscript, or in the decision to publish this review.
Figures
Similar articles
-
The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010-2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique.Insects. 2021 Apr 13;12(4):346. doi: 10.3390/insects12040346. Insects. 2021. PMID: 33924539 Free PMC article. Review.
-
Development of genetic sexing strains in Lepidoptera: from traditional to transgenic approaches.J Econ Entomol. 2005 Apr;98(2):248-59. doi: 10.1603/0022-0493-98.2.248. J Econ Entomol. 2005. PMID: 15889710
-
Molecular technologies to improve the effectiveness of the sterile insect technique.Genetica. 2011 Jan;139(1):1-5. doi: 10.1007/s10709-010-9543-z. Genetica. 2011. PMID: 21258957
-
Sterile Insect Technique (SIT) and Its Applications.Insects. 2021 Jul 13;12(7):638. doi: 10.3390/insects12070638. Insects. 2021. PMID: 34357298 Free PMC article.
-
Insect pathogens as biological control agents: Back to the future.J Invertebr Pathol. 2015 Nov;132:1-41. doi: 10.1016/j.jip.2015.07.009. Epub 2015 Jul 27. J Invertebr Pathol. 2015. PMID: 26225455 Review.
Cited by
-
Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management.Insects. 2024 Aug 30;15(9):653. doi: 10.3390/insects15090653. Insects. 2024. PMID: 39336621 Free PMC article. Review.
-
Verification of AKT and CDK5 Gene and RNA Interference Combined with Irradiation to Mediate Fertility Changes in Plutella xylostella (Linnaeus).Int J Mol Sci. 2024 Apr 24;25(9):4623. doi: 10.3390/ijms25094623. Int J Mol Sci. 2024. PMID: 38731841 Free PMC article.
-
Dichotomous sperm in Lepidopteran insects: a biorational target for pest management.Front Insect Sci. 2023 Aug 23;3:1198252. doi: 10.3389/finsc.2023.1198252. eCollection 2023. Front Insect Sci. 2023. PMID: 38469506 Free PMC article. Review.
-
Conservation of shibire and RpII215 temperature-sensitive lethal mutations between Drosophila and Bactrocera tryoni.Front Insect Sci. 2024 Mar 4;4:1249103. doi: 10.3389/finsc.2024.1249103. eCollection 2024. Front Insect Sci. 2024. PMID: 38469341 Free PMC article.
-
Effect of X-ray irradiation on the biological parameters of Xestia c-nigrum.Front Physiol. 2024 Feb 21;15:1362991. doi: 10.3389/fphys.2024.1362991. eCollection 2024. Front Physiol. 2024. PMID: 38449789 Free PMC article.
References
-
- Klassen W. Area-wide integrated pest management and the sterile insect technique. In: Dyck V.A., Hendrichs J., Robinson A.S., editors. Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management. Springer; Dordrecht, The Netherlands: 2005. pp. 39–68. - DOI
-
- Knipling E.F. The Basic Principles of Insect Suppression and Management. United States Department of Agriculture, Agricultural Research Service; Washington, DC, USA: 1979. Agricultural Handbook 512.
-
- Krafsur E.S. Sterile insect technique for suppressing and eradicating insect populations: 55 years and counting. J. Agric. Entomol. 1998;15:303–317.
-
- Vreysen M.J.B. Principles of area-wide integrated tsetse fly control using the sterile insect technique. Med. Trop. (Mars.) 2001;61:397–411. - PubMed
-
- Vreysen M.J.B., Robinson A.S. Ionising radiation and area-wide management of insect pests to promote sustainable agriculture. Agron. Sustain. Dev. 2011;31:233–250. doi: 10.1051/agro/2010009. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


