Revisiting Reverse Cholesterol Transport in the Context of High-Density Lipoprotein Free Cholesterol Bioavailability
- PMID: 31049149
- PMCID: PMC6489609
- DOI: 10.14797/mdcj-15-1-47
Revisiting Reverse Cholesterol Transport in the Context of High-Density Lipoprotein Free Cholesterol Bioavailability
Abstract
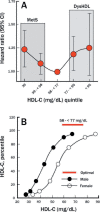
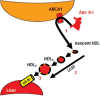
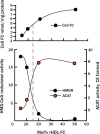
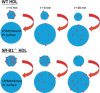
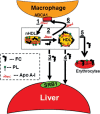
Dysregulated free cholesterol (FC) metabolism has been implicated in nearly all stages of atherosclerosis, the underlying cause of most cardiovascular disease. According to a widely cited model, the burden of macrophage FC in the arterial wall is relieved by transhepatic reverse cholesterol transport (RCT), which comprises three successive steps: (1) macrophage FC efflux to high-density lipoprotein (HDL) and/or its major protein, apolipoprotein AI; (2) FC esterification by lecithin:cholesterol acyltransferase (LCAT); and (3) HDL-cholesteryl ester (CE) uptake via the hepatic HDL-receptor, scavenger receptor class B type 1 (SR-B1). Recent studies have challenged the validity of this model, most notably the role of LCAT, which appears to be of minor importance. In mice, most macrophage-derived FC is rapidly cleared from plasma (t1/2 < 5 min) without esterification by hepatic uptake; the remainder is taken up by multiple tissue and cell types, especially erythrocytes. Further, some FC is cleared by the nonhepatic transintestinal pathway. Lastly, FC movement among lipid surfaces is reversible, so that a higher-than-normal level of HDL-FC bioavailability-defined by high plasma HDL levels concurrent with a high mol% HDL-FC-leads to the transfer of excess FC to cells in vivo. SR-B1-/- mice provide an animal model to study the mechanistic consequences of high HDL-FC bioavailability that provokes atherosclerosis and other metabolic abnormalities. Future efforts should aim to reduce HDL-FC bioavailability, thereby reducing FC accretion by tissues and the attendant atherosclerosis.
Keywords: atherogenesis; cholesterol; free cholesterol bioavailability; high-density lipoproteins; lipid metabolism; reverse cholesterol transport.
Conflict of interest statement
Conflict of Interest Disclosure: Dr. Gotto is a consultant for Esperion and KOWA Pharmaceuticals America, Inc. and is on the Data Safety Monitoring Board for Ionis Pharmaceuticals.
Figures
Similar articles
-
ABCA1-Derived Nascent High-Density Lipoprotein-Apolipoprotein AI and Lipids Metabolically Segregate.Arterioscler Thromb Vasc Biol. 2017 Dec;37(12):2260-2270. doi: 10.1161/ATVBAHA.117.310290. Epub 2017 Oct 26. Arterioscler Thromb Vasc Biol. 2017. PMID: 29074589 Free PMC article.
-
Rethinking reverse cholesterol transport and dysfunctional high-density lipoproteins.J Clin Lipidol. 2018 Jul-Aug;12(4):849-856. doi: 10.1016/j.jacl.2018.04.001. Epub 2018 Apr 12. J Clin Lipidol. 2018. PMID: 29731282 Free PMC article. Review.
-
Extended-Release Niacin/Laropiprant Improves Overall Efficacy of Postprandial Reverse Cholesterol Transport.Arterioscler Thromb Vasc Biol. 2016 Feb;36(2):285-94. doi: 10.1161/ATVBAHA.115.306834. Epub 2015 Dec 17. Arterioscler Thromb Vasc Biol. 2016. PMID: 26681758 Clinical Trial.
-
MicroRNA-24 aggravates atherosclerosis by inhibiting selective lipid uptake from HDL cholesterol via the post-transcriptional repression of scavenger receptor class B type I.Atherosclerosis. 2018 Mar;270:57-67. doi: 10.1016/j.atherosclerosis.2018.01.045. Epub 2018 Feb 4. Atherosclerosis. 2018. PMID: 29407889
-
HDL and Reverse Cholesterol Transport Biomarkers.Methodist Debakey Cardiovasc J. 2019 Jan-Mar;15(1):39-46. doi: 10.14797/mdcj-15-1-39. Methodist Debakey Cardiovasc J. 2019. PMID: 31049148 Free PMC article. Review.
Cited by
-
Association of Monocyte-to-HDL Cholesterol Ratio with Endothelial Dysfunction in Patients with Type 2 Diabetes.J Diabetes Res. 2024 Jan 11;2024:5287580. doi: 10.1155/2024/5287580. eCollection 2024. J Diabetes Res. 2024. PMID: 38239233 Free PMC article.
-
Different Pathways of Cellular Cholesterol Efflux.Cell Biochem Biophys. 2022 Sep;80(3):471-481. doi: 10.1007/s12013-022-01081-5. Epub 2022 Jun 23. Cell Biochem Biophys. 2022. PMID: 35737216 Review.
-
Free Cholesterol Bioavailability and Atherosclerosis.Curr Atheroscler Rep. 2022 May;24(5):323-336. doi: 10.1007/s11883-022-01011-z. Epub 2022 Mar 25. Curr Atheroscler Rep. 2022. PMID: 35332444 Free PMC article. Review.
-
Hyperalphalipoproteinemia and Beyond: The Role of HDL in Cardiovascular Diseases.Life (Basel). 2021 Jun 18;11(6):581. doi: 10.3390/life11060581. Life (Basel). 2021. PMID: 34207236 Free PMC article. Review.
-
Cholesterol efflux pathways, inflammation, and atherosclerosis.Crit Rev Biochem Mol Biol. 2021 Aug;56(4):426-439. doi: 10.1080/10409238.2021.1925217. Epub 2021 Jun 28. Crit Rev Biochem Mol Biol. 2021. PMID: 34182846 Free PMC article. Review.
References
-
- Anichkov NN. A history of experimentation on arterial atherosclerosis in animals. In: Blumenthal HT, editor. Cowdry's arteriosclerosis: a survey of the problem. 2nd ed. Springfield, IL: Charles C. Thomas Publishing; 1967. pp. 21–46. editor. p.
-
- Finking G, Hanke H. Nikolaj Nikolajewitsch Anitschkow (1885–1964) established the cholesterol-fed rabbit as a model for atherosclerosis research. Atherosclerosis. 1997 Nov;135(1):1–7. - PubMed
-
- Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966 Oct;34(4):679–97. - PubMed
-
- Castelli WP, Doyle JT, Gordon T et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977 May;55(5):767–72. - PubMed
-
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous


