Genetically-engineered pigs as sources for clinical red blood cell transfusion: What pathobiological barriers need to be overcome?
- PMID: 30711308
- PMCID: PMC6467751
- DOI: 10.1016/j.blre.2019.01.003
Genetically-engineered pigs as sources for clinical red blood cell transfusion: What pathobiological barriers need to be overcome?
Abstract
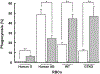
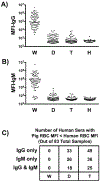
An alternative to human red blood cells (RBCs) for clinical transfusion would be advantageous, particularly in situations of massive acute blood loss (where availability and compatibility are limited) or chronic hematologic diseases requiring frequent transfusions (resulting in alloimmunization). Ideally, any alternative must be neither immunogenic nor pathogenic, but readily available, inexpensive, and physiologically effective. Pig RBCs (pRBCs) provide a promising alternative due to their several similarities with human RBCs, and our increasing ability to genetically-modify pigs to reduce cellular immunogenicity. We briefly summarize the history of xenotransfusion, the progress that has been made in recent years, and the remaining barriers. These barriers include prevention of (i) human natural antibody binding to pRBCs, (ii) their phagocytosis by macrophages, and (iii) the T cell adaptive immune response (in the absence of exogenous immunosuppressive therapy). Although techniques of genetic engineering have advanced in recent years, novel methods to introduce human transgenes into pRBCs (which do not have nuclei) will need to be developed before clinical trials can be initiated.
Keywords: Blood transfusion; Pig, genetically-engineered; Red blood cells; Sickle cell disease; Xenotransfusion; Xenotransplantation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest Statement
The authors declare that they have no relevant conflicts of interest.
Figures


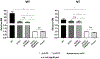
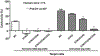
Similar articles
-
Investigation of the efficacy and safety of wild- type and triple-gene knockout pig RBC transfusions in nonhuman primates.Front Immunol. 2024 Jun 27;15:1418249. doi: 10.3389/fimmu.2024.1418249. eCollection 2024. Front Immunol. 2024. PMID: 38994362 Free PMC article.
-
Future prospects for the clinical transfusion of pig red blood cells.Blood Rev. 2023 Sep;61:101113. doi: 10.1016/j.blre.2023.101113. Epub 2023 Jul 14. Blood Rev. 2023. PMID: 37474379 Free PMC article. Review.
-
Genetically engineered pigs as a source for clinical red blood cell transfusion.Clin Lab Med. 2010 Jun;30(2):365-80. doi: 10.1016/j.cll.2010.02.001. Epub 2010 May 6. Clin Lab Med. 2010. PMID: 20513556
-
[Recent advance on blood group antigen modification of porcine erythrocytes].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2002 Jun;10(3):273-6. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2002. PMID: 12513803 Review. Chinese.
-
The immense potential of xenotransplantation in surgery.Int J Surg. 2011;9(2):122-9. doi: 10.1016/j.ijsu.2010.11.002. Epub 2010 Nov 5. Int J Surg. 2011. PMID: 21059418 Review.
Cited by
-
Investigation of the efficacy and safety of wild- type and triple-gene knockout pig RBC transfusions in nonhuman primates.Front Immunol. 2024 Jun 27;15:1418249. doi: 10.3389/fimmu.2024.1418249. eCollection 2024. Front Immunol. 2024. PMID: 38994362 Free PMC article.
-
Initial investigation on the feasibility of porcine red blood cells from genetically modified pigs as an alternative to human red blood cells for transfusion.Front Immunol. 2023 Nov 14;14:1298035. doi: 10.3389/fimmu.2023.1298035. eCollection 2023. Front Immunol. 2023. PMID: 38035112 Free PMC article.
-
Challenge Inoculum for Hepatitis C Virus Controlled Human Infection Model.Clin Infect Dis. 2023 Aug 14;77(Suppl 3):S257-S261. doi: 10.1093/cid/ciad336. Clin Infect Dis. 2023. PMID: 37579208 Free PMC article.
-
Future prospects for the clinical transfusion of pig red blood cells.Blood Rev. 2023 Sep;61:101113. doi: 10.1016/j.blre.2023.101113. Epub 2023 Jul 14. Blood Rev. 2023. PMID: 37474379 Free PMC article. Review.
-
Initial experimental experience of triple-knockout pig red blood cells as potential sources for transfusion in alloimmunized patients with sickle cell disease.Transfusion. 2021 Nov;61(11):3104-3118. doi: 10.1111/trf.16667. Epub 2021 Sep 22. Transfusion. 2021. PMID: 34553390 Free PMC article.
References
-
- Global status report on blood safety and availability 2016. Geneva: World Health Organization; 2017.
-
- American Red Cross. Blood needs and blood supply. RedCrossBlood.Org 2018. Accessed Apr 28, 2018.
-
- Center for Biologics Evaluation and Research. Donating blood questions and answers. U S Food and Drug Administration Home Page. https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/Questionsa... 2018. Accessed Apr 28, 2018.
-
- Revised preventative measures to reduce the possible risk of transmission of Creutzfeldt-Jakob disease and variant Creutzfeld-Jakob disease by blood and blood products. US FDA-US Department of Health and Human Services; https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceR... 2018. Accessed Jun 24, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


