Two breakthrough gene-targeted treatments for spinal muscular atrophy: challenges remain
- PMID: 29985170
- PMCID: PMC6063504
- DOI: 10.1172/JCI121658
Two breakthrough gene-targeted treatments for spinal muscular atrophy: challenges remain
Abstract
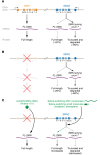
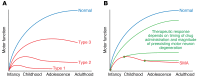
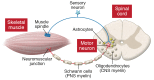
The motor neuron disease spinal muscular atrophy (SMA) is caused by recessive, loss-of-function mutations of the survival motor neuron 1 gene (SMN1). Alone, such mutations are embryonically lethal, but SMA patients retain a paralog gene, SMN2, that undergoes alternative pre-mRNA splicing, producing low levels of SMN protein. By mechanisms that are not well understood, reduced expression of the ubiquitously expressed SMN protein causes an early-onset motor neuron disease that often results in infantile or childhood mortality. Recently, striking clinical improvements have resulted from two novel treatment strategies to increase SMN protein by (a) modulating the splicing of existing SMN2 pre-mRNAs using antisense oligonucleotides, and (b) transducing motor neurons with self-complementary adeno-associated virus 9 (scAAV9) expressing exogenous SMN1 cDNA. We review the recently published clinical trial results and discuss the differing administration, tissue targeting, and potential toxicities of these two therapies. We also focus on the challenges that remain, emphasizing the many clinical and biologic questions that remain open. Answers to these questions will enable further optimization of these remarkable SMA treatments as well as provide insights that may well be useful in application of these therapeutic platforms to other diseases.
Conflict of interest statement
Figures
Similar articles
-
The Antisense Transcript SMN-AS1 Regulates SMN Expression and Is a Novel Therapeutic Target for Spinal Muscular Atrophy.Neuron. 2017 Jan 4;93(1):66-79. doi: 10.1016/j.neuron.2016.11.033. Epub 2016 Dec 22. Neuron. 2017. PMID: 28017471 Free PMC article.
-
Normalization of Patient-Identified Plasma Biomarkers in SMNΔ7 Mice following Postnatal SMN Restoration.PLoS One. 2016 Dec 1;11(12):e0167077. doi: 10.1371/journal.pone.0167077. eCollection 2016. PLoS One. 2016. PMID: 27907033 Free PMC article.
-
Spinal muscular atrophy: antisense oligonucleotide therapy opens the door to an integrated therapeutic landscape.Hum Mol Genet. 2017 Oct 1;26(R2):R151-R159. doi: 10.1093/hmg/ddx215. Hum Mol Genet. 2017. PMID: 28977438 Review.
-
Heat increases full-length SMN splicing: promise for splice-augmenting therapies for SMA.Hum Genet. 2022 Feb;141(2):239-256. doi: 10.1007/s00439-021-02408-7. Epub 2022 Jan 28. Hum Genet. 2022. PMID: 35088120
-
Recent Progress in Gene-Targeting Therapies for Spinal Muscular Atrophy: Promises and Challenges.Genes (Basel). 2024 Jul 30;15(8):999. doi: 10.3390/genes15080999. Genes (Basel). 2024. PMID: 39202360 Free PMC article. Review.
Cited by
-
New sights on long non-coding RNAs in glioblastoma: A review of molecular mechanism.Heliyon. 2024 Oct 23;10(21):e39744. doi: 10.1016/j.heliyon.2024.e39744. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553554 Free PMC article. Review.
-
Spinal Muscular Atrophy: Current Medications and Re-purposed Drugs.Cell Mol Neurobiol. 2024 Nov 8;44(1):75. doi: 10.1007/s10571-024-01511-3. Cell Mol Neurobiol. 2024. PMID: 39514016 Free PMC article. Review.
-
Current developments of gene therapy in human diseases.MedComm (2020). 2024 Aug 16;5(9):e645. doi: 10.1002/mco2.645. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39156766 Free PMC article. Review.
-
A rapid and easy-to-use spinal muscular atrophy screening tool based on primers with high specificity and amplification efficiency for SMN1 combined with single-stranded tag hybridization assay.PLoS One. 2024 Aug 1;19(8):e0308179. doi: 10.1371/journal.pone.0308179. eCollection 2024. PLoS One. 2024. PMID: 39088538 Free PMC article.
-
A Five-Year Review of Newborn Screening for Spinal Muscular Atrophy in the State of Utah: Lessons Learned.Int J Neonatal Screen. 2024 Jul 22;10(3):54. doi: 10.3390/ijns10030054. Int J Neonatal Screen. 2024. PMID: 39051410 Free PMC article.
References
-
- Werdnig G. Zwei frühinfantile hereditäre fälle von progressive muskelatrophie unter dem bilde der dystrophie, aber auf neurotischer grundlage. Arch fur Psychiatr Nervenkrankh. 1891;22:437–481. doi: 10.1007/BF01776636. - DOI
-
- Parsons DW, McAndrew PE, Iannaccone ST, Mendell JR, Burghes AH, Prior TW. Intragenic telSMN mutations: frequency, distribution, evidence of a founder effect, and modification of the spinal muscular atrophy phenotype by cenSMN copy number. Am J Hum Genet. 1998;63(6):1712–1723. doi: 10.1086/302160. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


