A twist defect mechanism for ATP-dependent translocation of nucleosomal DNA
- PMID: 29809147
- PMCID: PMC6031429
- DOI: 10.7554/eLife.34100
A twist defect mechanism for ATP-dependent translocation of nucleosomal DNA
Abstract
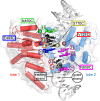
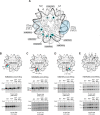
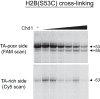
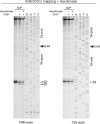
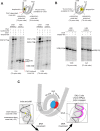
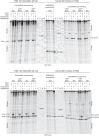
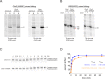
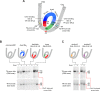
As superfamily 2 (SF2)-type translocases, chromatin remodelers are expected to use an inchworm-type mechanism to walk along DNA. Yet how they move DNA around the histone core has not been clear. Here we show that a remodeler ATPase motor can shift large segments of DNA by changing the twist and length of nucleosomal DNA at superhelix location 2 (SHL2). Using canonical and variant 601 nucleosomes, we find that the Saccharomyces cerevisiae Chd1 remodeler decreased DNA twist at SHL2 in nucleotide-free and ADP-bound states, and increased twist with transition state analogs. These differences in DNA twist allow the open state of the ATPase to pull in ~1 base pair (bp) by stabilizing a small DNA bulge, and closure of the ATPase to shift the DNA bulge toward the dyad. We propose that such formation and elimination of twist defects underlie the mechanism of nucleosome sliding by CHD-, ISWI-, and SWI/SNF-type remodelers.
Keywords: DNA translocation; S. cerevisiae; Snf2 ATPase; biochemistry; chemical biology; chromatin remodeling; chromosomes; gene expression; nucleosome; superfamily 2 ATPase; twist defect; xenopus.
© 2018, Winger et al.
Conflict of interest statement
JW, IN, RL, GB No competing interests declared
Figures













Similar articles
-
The Chd1 chromatin remodeler forms long-lived complexes with nucleosomes in the presence of ADP·BeF3- and transition state analogs.J Biol Chem. 2019 Nov 29;294(48):18181-18191. doi: 10.1074/jbc.RA119.009782. Epub 2019 Oct 21. J Biol Chem. 2019. PMID: 31636125 Free PMC article.
-
Autoinhibitory elements of the Chd1 remodeler block initiation of twist defects by destabilizing the ATPase motor on the nucleosome.Proc Natl Acad Sci U S A. 2021 Jan 26;118(4):e2014498118. doi: 10.1073/pnas.2014498118. Proc Natl Acad Sci U S A. 2021. PMID: 33468676 Free PMC article.
-
Chromatin remodeling by ISW2 and SWI/SNF requires DNA translocation inside the nucleosome.Nat Struct Mol Biol. 2006 Apr;13(4):339-46. doi: 10.1038/nsmb1071. Epub 2006 Mar 5. Nat Struct Mol Biol. 2006. PMID: 16518397
-
Biophysics of Chromatin Remodeling.Annu Rev Biophys. 2021 May 6;50:73-93. doi: 10.1146/annurev-biophys-082520-080201. Epub 2021 Jan 4. Annu Rev Biophys. 2021. PMID: 33395550 Free PMC article. Review.
-
Mechanisms of ATP-dependent nucleosome sliding.Curr Opin Struct Biol. 2010 Feb;20(1):73-81. doi: 10.1016/j.sbi.2009.12.002. Epub 2010 Jan 8. Curr Opin Struct Biol. 2010. PMID: 20060707 Free PMC article. Review.
Cited by
-
Sequence Dependence in Nucleosome Dynamics.J Phys Chem B. 2024 Apr 4;128(13):3090-3101. doi: 10.1021/acs.jpcb.3c07363. Epub 2024 Mar 26. J Phys Chem B. 2024. PMID: 38530903 Free PMC article.
-
Energy-driven genome regulation by ATP-dependent chromatin remodellers.Nat Rev Mol Cell Biol. 2024 Apr;25(4):309-332. doi: 10.1038/s41580-023-00683-y. Epub 2023 Dec 11. Nat Rev Mol Cell Biol. 2024. PMID: 38081975 Review.
-
Bi-directional nucleosome sliding by the Chd1 chromatin remodeler integrates intrinsic sequence-dependent and ATP-dependent nucleosome positioning.Nucleic Acids Res. 2023 Oct 27;51(19):10326-10343. doi: 10.1093/nar/gkad738. Nucleic Acids Res. 2023. PMID: 37738162 Free PMC article.
-
Structural Plasticity of Pioneer Factor Sox2 and DNA Bendability Modulate Nucleosome Engagement and Sox2-Oct4 Synergism.J Mol Biol. 2023 Jan 30;435(2):167916. doi: 10.1016/j.jmb.2022.167916. Epub 2022 Dec 7. J Mol Biol. 2023. PMID: 36495920 Free PMC article.
-
H2A.Z deposition by SWR1C involves multiple ATP-dependent steps.Nat Commun. 2022 Nov 17;13(1):7052. doi: 10.1038/s41467-022-34861-x. Nat Commun. 2022. PMID: 36396651 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials


