Rethinking reverse cholesterol transport and dysfunctional high-density lipoproteins
- PMID: 29731282
- PMCID: PMC6064676
- DOI: 10.1016/j.jacl.2018.04.001
Rethinking reverse cholesterol transport and dysfunctional high-density lipoproteins
Abstract
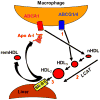
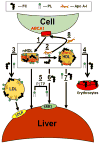
Human plasma high-density lipoprotein cholesterol concentrations are a negative risk factor for atherosclerosis-linked cardiovascular disease. Pharmacological attempts to reduce atherosclerotic cardiovascular disease by increasing plasma high-density lipoprotein cholesterol have been disappointing so that recent research has shifted from HDL quantity to HDL quality, that is, functional vs dysfunctional HDL. HDL has varying degrees of dysfunction reflected in impaired reverse cholesterol transport (RCT). In the context of atheroprotection, RCT occurs by 2 mechanisms: one is the well-known trans-hepatic pathway comprising macrophage free cholesterol (FC) efflux, which produces early forms of FC-rich nascent HDL (nHDL). Lecithin:cholesterol acyltransferase converts HDL-FC to HDL-cholesteryl ester while converting nHDL from a disc to a mature spherical HDL, which transfers its cholesteryl ester to the hepatic HDL receptor, scavenger receptor B1 for uptake, conversion to bile salts, or transfer to the intestine for excretion. Although widely cited, current evidence suggests that this is a minor pathway and that most HDL-FC and nHDL-FC rapidly transfer directly to the liver independent of lecithin:cholesterol acyltransferase activity. A small fraction of plasma HDL-FC enters the trans-intestinal efflux pathway comprising direct FC transfer to the intestine. SR-B1-/- mice, which have impaired trans-hepatic FC transport, are characterized by high plasma levels of a dysfunctional FC-rich HDL that increases plasma FC bioavailability in a way that produces whole-body hypercholesterolemia and multiple pathologies. The design of future therapeutic strategies to improve RCT will have to be formulated in the context of these dual RCT mechanisms and the role of FC bioavailability.
Keywords: ATP-binding cassette transporter A1; Atherogenesis; Cholesterol bioavailability; HDL biogenesis; Lipoprotein receptors; Reverse cholesterol transport.
Copyright © 2018 National Lipid Association. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Revisiting Reverse Cholesterol Transport in the Context of High-Density Lipoprotein Free Cholesterol Bioavailability.Methodist Debakey Cardiovasc J. 2019 Jan-Mar;15(1):47-54. doi: 10.14797/mdcj-15-1-47. Methodist Debakey Cardiovasc J. 2019. PMID: 31049149 Free PMC article. Review.
-
ABCA1-Derived Nascent High-Density Lipoprotein-Apolipoprotein AI and Lipids Metabolically Segregate.Arterioscler Thromb Vasc Biol. 2017 Dec;37(12):2260-2270. doi: 10.1161/ATVBAHA.117.310290. Epub 2017 Oct 26. Arterioscler Thromb Vasc Biol. 2017. PMID: 29074589 Free PMC article.
-
Hepatic Overexpression of Endothelial Lipase Lowers High-Density Lipoprotein but Maintains Reverse Cholesterol Transport in Mice: Role of Scavenger Receptor Class B Type I/ATP-Binding Cassette Transporter A1-Dependent Pathways.Arterioscler Thromb Vasc Biol. 2018 Jul;38(7):1454-1467. doi: 10.1161/ATVBAHA.118.311056. Epub 2018 May 10. Arterioscler Thromb Vasc Biol. 2018. PMID: 29748333 Free PMC article.
-
Free Cholesterol Bioavailability and Atherosclerosis.Curr Atheroscler Rep. 2022 May;24(5):323-336. doi: 10.1007/s11883-022-01011-z. Epub 2022 Mar 25. Curr Atheroscler Rep. 2022. PMID: 35332444 Free PMC article. Review.
-
Age-associated decrease of high-density lipoprotein-mediated reverse cholesterol transport activity.Rejuvenation Res. 2009 Apr;12(2):117-26. doi: 10.1089/rej.2009.0840. Rejuvenation Res. 2009. PMID: 19405812 Review.
Cited by
-
Very high high-density lipoprotein cholesterol may be associated with higher risk of cognitive impairment in older adults.Nutr J. 2024 Jul 17;23(1):79. doi: 10.1186/s12937-024-00983-9. Nutr J. 2024. PMID: 39020341 Free PMC article.
-
Causal association between lipoproteins and risk of coronary artery disease-a systematic review and meta-analysis of Mendelian randomization studies.Clin Res Cardiol. 2024 Feb 26. doi: 10.1007/s00392-024-02420-7. Online ahead of print. Clin Res Cardiol. 2024. PMID: 38407584 Review.
-
Identification of the specific molecular and functional signatures of pre-beta-HDL: relevance to cardiovascular disease.Basic Res Cardiol. 2023 Aug 28;118(1):33. doi: 10.1007/s00395-023-01004-2. Basic Res Cardiol. 2023. PMID: 37639039
-
The Lipid Energy Model: Reimagining Lipoprotein Function in the Context of Carbohydrate-Restricted Diets.Metabolites. 2022 May 20;12(5):460. doi: 10.3390/metabo12050460. Metabolites. 2022. PMID: 35629964 Free PMC article.
-
Is it time to change the goals of lipid management in type 1 diabetes mellitus? Changes in apolipoprotein levels during the first year of type 1 diabetes mellitus. Prospective InLipoDiab1 study.Arch Med Sci. 2020 Oct 29;18(3):596-603. doi: 10.5114/aoms.2020.100255. eCollection 2022. Arch Med Sci. 2020. PMID: 35591821 Free PMC article.
References
-
- Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–9. - PubMed
-
- Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–67. - PubMed
-
- Glomset JA, Wright JL. Some Properties of a Cholesterol Esterifying Enzyme in Human Plasma. Biochim Biophys Acta. 1964;89:266–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous


