Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study
- PMID: 29716436
- PMCID: PMC6460686
- DOI: 10.1177/1352458518771875
Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study
Abstract
Background: In the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study, Cladribine Tablets significantly improved clinical and magnetic resonance imaging (MRI) outcomes (vs placebo) in patients with relapsing-remitting multiple sclerosis.
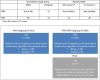
Objective: Describe two clinically relevant definitions for patients with high disease activity (HDA) at baseline of the CLARITY study (utility verified in patients receiving placebo) and assess the treatment effects of Cladribine Tablets 3.5 mg/kg compared with the overall study population.
Methods: Outcomes of patients randomised to Cladribine Tablets 3.5 mg/kg or placebo were analysed for subgroups using HDA definitions based on high relapse activity (HRA; patients with ⩾2 relapses during the year prior to study entry, whether on DMD treatment or not) or HRA plus disease activity on treatment (HRA + DAT; patients with ⩾2 relapses during the year prior to study entry, whether on DMD treatment or not, PLUS patients with ⩾1 relapse during the year prior to study entry while on therapy with other DMDs and ⩾1 T1 Gd+ or ⩾9 T2 lesions).
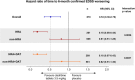
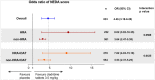
Results: In the overall population, Cladribine Tablets 3.5 mg/kg reduced the risk of 6-month-confirmed Expanded Disability Status Scale (EDSS) worsening by 47% vs placebo. A risk reduction of 82% vs placebo was seen in both the HRA and HRA + DAT subgroups (vs 19% for non-HRA and 18% for non-HRA + DAT), indicating greater responsiveness to Cladribine Tablets 3.5 mg/kg in patients with HDA. There were consistent results for other efficacy endpoints. The safety profile in HDA patients was consistent with the overall CLARITY population.
Conclusion: Patients with HDA showed clinical and MRI responses to Cladribine Tablets 3.5 mg/kg that were generally better than, or at least comparable with, the outcomes seen in the overall CLARITY population.
Keywords: Cladribine Tablets; efficacy; high disease activity; risk:benefit; safety.
Conflict of interest statement
Figures


Similar articles
-
Efficacy of cladribine tablets in high disease activity patients with relapsing multiple sclerosis: post hoc analysis of subgroups with and without prior disease-modifying drug treatment.Curr Med Res Opin. 2021 Mar;37(3):459-464. doi: 10.1080/03007995.2020.1865888. Epub 2021 Feb 1. Curr Med Res Opin. 2021. PMID: 33331183
-
Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis.Lancet Neurol. 2011 Apr;10(4):329-37. doi: 10.1016/S1474-4422(11)70023-0. Lancet Neurol. 2011. PMID: 21397565
-
Long-Term Disease Stability Assessed by the Expanded Disability Status Scale in Patients Treated with Cladribine Tablets 3.5 mg/kg for Relapsing Multiple Sclerosis: An Exploratory Post Hoc Analysis of the CLARITY and CLARITY Extension Studies.Adv Ther. 2021 Sep;38(9):4975-4985. doi: 10.1007/s12325-021-01865-w. Epub 2021 Aug 9. Adv Ther. 2021. PMID: 34370275 Free PMC article. Clinical Trial.
-
Cladribine tablets: in relapsing-remitting multiple sclerosis.CNS Drugs. 2011 Mar;25(3):239-49. doi: 10.2165/11204740-000000000-00000. CNS Drugs. 2011. PMID: 21323395 Review.
-
Cladribine Tablets: A Review in Relapsing MS.CNS Drugs. 2018 Aug;32(8):785-796. doi: 10.1007/s40263-018-0562-0. CNS Drugs. 2018. PMID: 30105527 Free PMC article. Review.
Cited by
-
Early clinical effect of cladribine in patients with highly active multiple sclerosis in Mexico.Mult Scler J Exp Transl Clin. 2024 Aug 1;10(3):20552173241260156. doi: 10.1177/20552173241260156. eCollection 2024 Jul-Sep. Mult Scler J Exp Transl Clin. 2024. PMID: 39091340 Free PMC article.
-
Real-World Effectiveness and Safety of Cladribine in Multiple Sclerosis: Longitudinal Data From the Nationwide Registry in Argentina.Clin Neuropharmacol. 2024 Jul-Aug 01;47(4):120-127. doi: 10.1097/WNF.0000000000000598. Epub 2024 May 22. Clin Neuropharmacol. 2024. PMID: 39008542
-
Magnetic Resonance Imaging Evidence Supporting the Efficacy of Cladribine Tablets in the Treatment of Relapsing-Remitting Multiple Sclerosis.CNS Drugs. 2024 Apr;38(4):267-279. doi: 10.1007/s40263-024-01074-3. Epub 2024 Mar 15. CNS Drugs. 2024. PMID: 38489020 Free PMC article. Review.
-
Holistic, Long-Term Management of People with Relapsing Multiple Sclerosis with Cladribine Tablets: Expert Opinion from France.Neurol Ther. 2024 Jun;13(3):503-518. doi: 10.1007/s40120-024-00589-7. Epub 2024 Mar 15. Neurol Ther. 2024. PMID: 38488979 Free PMC article. Review.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article. Review.
References
-
- Chiu AW, Richert N, Ehrmantraut M, et al. Heterogeneity in response to interferon beta in patients with multiple sclerosis: A 3-year monthly imaging study. Arch Neurol 2009; 66: 39–43. - PubMed
-
- Mahurkar S, Suppiah V, O’Doherty C. Pharmacogenomics of interferon beta and glatiramer acetate response: A review of the literature. Autoimmun Rev 2014; 13: 178–186. - PubMed
-
- Shirani A, Zhao Y, Karim ME, et al. Investigation of heterogeneity in the association between interferon beta and disability progression in multiple sclerosis: An observational study. Eur J Neurol 2014; 21: 835–844. - PubMed
-
- Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 2013; 73: 95–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources


