Effect of developmental NMDAR antagonism with CGP 39551 on aspartame-induced hypothalamic and adrenal gene expression
- PMID: 29561882
- PMCID: PMC5862471
- DOI: 10.1371/journal.pone.0194416
Effect of developmental NMDAR antagonism with CGP 39551 on aspartame-induced hypothalamic and adrenal gene expression
Abstract
Rationale: Aspartame (L-aspartyl phenylalanine methyl ester) is a non-nutritive sweetener (NNS) approved for use in more than 6000 dietary products and pharmaceuticals consumed by the general public including adults and children, pregnant and nursing mothers. However a recent prospective study reported a doubling of the risk of being overweight amongst 1-year old children whose mothers consumed NNS-sweetened beverages daily during pregnancy. We have previously shown that chronic aspartame (ASP) exposure commencing in utero may detrimentally affect adulthood adiposity status, glucose metabolism and aspects of behavior and spatial cognition, and that this can be modulated by developmental N-methyl-D-aspartate receptor (NMDAR) blockade with the competitive antagonist CGP 39551 (CGP). Since glucose homeostasis and certain aspects of behavior and locomotion are regulated in part by the NMDAR-rich hypothalamus, which is part of the hypothalamic-pituitary-adrenal- (HPA) axis, we have elected to examine changes in hypothalamic and adrenal gene expression in response to ASP exposure in the presence or absence of developmental NMDAR antagonism with CGP, using Affymetrix microarray analysis.
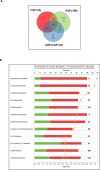
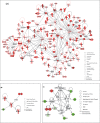
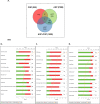
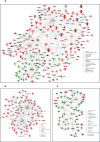
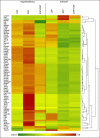
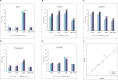
Results: Using 2-factor ANOVA we identified 189 ASP-responsive differentially expressed genes (DEGs) in the adult male hypothalamus and 2188 in the adrenals, and a further 23 hypothalamic and 232 adrenal genes significantly regulated by developmental treatment with CGP alone. ASP exposure robustly elevated the expression of a network of genes involved in hypothalamic neurosteroidogenesis, together with cell stress and inflammatory genes, consistent with previous reports of aspartame-induced CNS stress and oxidative damage. These genes were not differentially expressed in ASP mice with CGP antagonism. In the adrenal glands of ASP-exposed mice, GABA and Glutamate receptor subunit genes were amongst those most highly upregulated. Developmental NMDAR antagonism alone had less effect on adulthood gene expression and affected mainly hypothalamic neurogenesis and adrenal steroid metabolism. Combined ASP + CGP treatment mainly upregulated genes involved in adrenal drug and cholesterol metabolism.
Conclusion: ASP exposure increased the expression of functional networks of genes involved in hypothalamic neurosteroidogenesis and adrenal catecholamine synthesis, patterns of expression which were not present in ASP-exposed mice with developmental NMDAR antagonism.
Conflict of interest statement
Figures
Similar articles
-
Differential effects of early-life NMDA receptor antagonism on aspartame-impaired insulin tolerance and behavior.Physiol Behav. 2016 Dec 1;167:209-221. doi: 10.1016/j.physbeh.2016.09.011. Epub 2016 Sep 15. Physiol Behav. 2016. PMID: 27640132
-
The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis.Physiol Behav. 2014 Jan 30;124:77-91. doi: 10.1016/j.physbeh.2013.10.035. Epub 2013 Oct 31. Physiol Behav. 2014. PMID: 24184413
-
Food intake-induced leptin secretion modulates hypothalamo-pituitary-adrenal axis response and hypothalamic Ob-Rb expression to insulin administration.Neuroendocrinology. 2000 Dec;72(6):341-9. doi: 10.1159/000054603. Neuroendocrinology. 2000. PMID: 11146417
-
Prenatal xenobiotic exposure and intrauterine hypothalamus-pituitary-adrenal axis programming alteration.Toxicology. 2014 Nov 5;325:74-84. doi: 10.1016/j.tox.2014.08.015. Epub 2014 Sep 4. Toxicology. 2014. PMID: 25194749 Review.
-
CGP 37849 and CGP 39551: novel competitive N-methyl-D-aspartate receptor antagonists with potent oral anticonvulsant activity.Prog Clin Biol Res. 1990;361:421-7. Prog Clin Biol Res. 1990. PMID: 1981260 Review. No abstract available.
Cited by
-
Daily Early-Life Exposures to Diet Soda and Aspartame Are Associated with Autism in Males: A Case-Control Study.Nutrients. 2023 Aug 29;15(17):3772. doi: 10.3390/nu15173772. Nutrients. 2023. PMID: 37686804 Free PMC article.
-
Learning and memory deficits produced by aspartame are heritable via the paternal lineage.Sci Rep. 2023 Aug 31;13(1):14326. doi: 10.1038/s41598-023-41213-2. Sci Rep. 2023. PMID: 37652922 Free PMC article.
-
The impact of non-caloric artificial sweetener aspartame on female reproductive system in mice model.Reprod Biol Endocrinol. 2023 Aug 14;21(1):73. doi: 10.1186/s12958-023-01115-4. Reprod Biol Endocrinol. 2023. PMID: 37580716 Free PMC article.
-
Hippocampal Changes Elicited by Metabolic and Inflammatory Stressors following Prenatal Maternal Infection.Genes (Basel). 2022 Dec 26;14(1):77. doi: 10.3390/genes14010077. Genes (Basel). 2022. PMID: 36672818 Free PMC article.
-
Transgenerational transmission of aspartame-induced anxiety and changes in glutamate-GABA signaling and gene expression in the amygdala.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2213120119. doi: 10.1073/pnas.2213120119. Epub 2022 Dec 2. Proc Natl Acad Sci U S A. 2022. PMID: 36459641 Free PMC article.
References
-
- Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012; 112(5):739–58. doi: 10.1016/j.jand.2012.03.009 - DOI - PubMed
-
- Soffritti M, Padovani M, Tibaldi E, Falcioni L, Manservisi F, Belpoggi F. The carcinogenic effects of aspartame: The urgent need for regulatory re-evaluation. Am J Ind Med. 2014; 57(4):383–97. doi: 10.1002/ajim.22296 - DOI - PubMed
-
- Araújo JR, Martel F, Keating E. Exposure to non-nutritive sweeteners during pregnancy and lactation: Impact in programming of metabolic diseases in the progeny later in life. Reprod Toxicol. 2014; 49:196–201. doi: 10.1016/j.reprotox.2014.09.007 - DOI - PubMed
-
- Azad MB, Sharma AK, de Souza RJ, Dolinsky VW, Becker AB, Mandhane PJ et al. Canadian Healthy Infant Longitudinal Development Study Investigators. Association Between Artificially Sweetened Beverage Consumption During Pregnancy and Infant Body Mass Index. JAMA Pediatr. 2016; 170(7):662–70. doi: 10.1001/jamapediatrics.2016.0301 - DOI - PubMed
-
- Collison KS, Makhoul NJ, Zaidi MZ, Saleh SM, Andres B, Inglis A et al. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One. 2012; 7 (4): e31570 doi: 10.1371/journal.pone.0031570 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


