Selective Sparing of Striatal Interneurons after Poly (ADP-Ribose) Polymerase 1 Inhibition in the R6/2 Mouse Model of Huntington's Disease
- PMID: 28824383
- PMCID: PMC5539174
- DOI: 10.3389/fnana.2017.00061
Selective Sparing of Striatal Interneurons after Poly (ADP-Ribose) Polymerase 1 Inhibition in the R6/2 Mouse Model of Huntington's Disease
Abstract
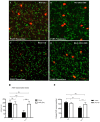
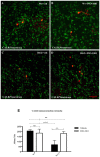
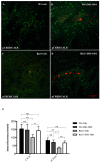
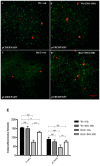
Poly (ADP-ribose) polymerases (PARPs) are enzymes that catalyze ADP-ribose units transfer from NAD to their substrate proteins. It has been observed that PARP-1 is able to increase both post-ischemic and excitotoxic neuronal death. In fact, we have previously shown that, INO-1001, a PARP-1 inhibitor, displays a neuroprotective effect in the R6/2 model of Huntington's disease (HD). In this study, we investigated the effects of PARP-1-inhibition on modulation of phosphorylated c-AMP response element binding protein (pCREB) and CREB-binding protein (CBP) localization in the different striatal neuronal subsets. Moreover, we studied the neurodegeneration of those interneurons that are particularly vulnerable to HD such as parvalbuminergic and calretininergic, and of other subclasses of interneurons that are known to be resistant, such as cholinergic and somatostatinergic interneurons. Transgenic mice were treated with INO-1001 (10 mg/Kg daily) starting from 4 weeks of age. Double-label immunofluorescence was performed to value the distribution of CBP in ubiquitinated Neuronal intranuclear inclusions (NIIs) in the striatum. INO-1001-treated and saline-treated brain sections were incubated with: goat anti-choline acetyl transferase; goat anti-nitric oxide synthase; mouse anti-parvalbumin and mouse anti-calretinin. Morphometric evaluation and cell counts were performed. Our study showed that the PARP inhibitor has a positive effect in sparing parvalbumin and calretinin-containing interneurons of the striatum, where CREB was upregulated. Moreover, INO-1001 promoted CBP localization into the nuclei of the R6/2 mouse. The sum of our data corroborates the previous observations indicating PARP inhibition as a possible therapeutic tool to fight HD.
Keywords: CBP; Huntington’s disease; PARP-1 inhibition; calretinin; interneurons; neurodegeneration; pCREB; parvalbumin.
Figures


Similar articles
-
Phosphodiesterase type IV inhibition prevents sequestration of CREB binding protein, protects striatal parvalbumin interneurons and rescues motor deficits in the R6/2 mouse model of Huntington's disease.Eur J Neurosci. 2009 Mar;29(5):902-10. doi: 10.1111/j.1460-9568.2009.06649.x. Eur J Neurosci. 2009. PMID: 19291221
-
PARP-1 Inhibition Is Neuroprotective in the R6/2 Mouse Model of Huntington's Disease.PLoS One. 2015 Aug 7;10(8):e0134482. doi: 10.1371/journal.pone.0134482. eCollection 2015. PLoS One. 2015. PMID: 26252217 Free PMC article.
-
Cellular localization and development of neuronal intranuclear inclusions in striatal and cortical neurons in R6/2 transgenic mice.J Comp Neurol. 2002 Jul 29;449(3):241-69. doi: 10.1002/cne.10295. J Comp Neurol. 2002. PMID: 12115678
-
Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage.Curr Opin Pharmacol. 2008 Feb;8(1):96-103. doi: 10.1016/j.coph.2007.10.005. Epub 2007 Nov 26. Curr Opin Pharmacol. 2008. PMID: 18032109 Review.
-
Poly(ADP-ribose)polymerase inhibition - where now?Curr Med Chem. 2005;12(20):2373-92. doi: 10.2174/0929867054864778. Curr Med Chem. 2005. PMID: 16181138 Review.
Cited by
-
Poly ADP-ribose signaling is dysregulated in Huntington disease.Proc Natl Acad Sci U S A. 2024 Oct;121(40):e2318098121. doi: 10.1073/pnas.2318098121. Epub 2024 Sep 27. Proc Natl Acad Sci U S A. 2024. PMID: 39331414
-
Nicotinamide adenine dinucleotide treatment confers resistance to neonatal ischemia and hypoxia: effects on neurobehavioral phenotypes.Neural Regen Res. 2024 Dec 1;19(12):2760-2772. doi: 10.4103/NRR.NRR-D-23-01490. Epub 2024 Mar 1. Neural Regen Res. 2024. PMID: 38595293 Free PMC article.
-
Parthanatos: Mechanisms, modulation, and therapeutic prospects in neurodegenerative disease and stroke.Biochem Pharmacol. 2024 Oct;228:116174. doi: 10.1016/j.bcp.2024.116174. Epub 2024 Mar 27. Biochem Pharmacol. 2024. PMID: 38552851 Review.
-
Unlocking the epigenetic symphony: histone acetylation's impact on neurobehavioral change in neurodegenerative disorders.Epigenomics. 2024 Mar;16(5):331-358. doi: 10.2217/epi-2023-0428. Epub 2024 Feb 7. Epigenomics. 2024. PMID: 38321930 Review.
-
Transcriptional and Histone Acetylation Changes Associated with CRE Elements Expose Key Factors Governing the Regulatory Circuit in the Early Stage of Huntington's Disease Models.Int J Mol Sci. 2023 Jun 29;24(13):10848. doi: 10.3390/ijms241310848. Int J Mol Sci. 2023. PMID: 37446028 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


